Advertisement
Table of Contents
- 1 Table of Contents
- 2 Application, Safety Information
- 3 Controls and Indicators
- 4 Setup
- 5 Application
- 6 Data Output
- 7 Error Codes
- 8 Software Installation
- 9 Cleaning, Maintenance, Disposal
- 10 Technical Specifications
- 11 10 Order Information
- 12 Appendix - Electromagnetic Compatibility (EMC)
- Download this manual
Advertisement
Table of Contents

Summary of Contents for GE TONOPORT VI
- Page 1 GE Healthcare TONOPORT VI Ambulatory Blood Pressure System Firmware Version 3.0 Operator's Manual 2001589-312 ENG Revision B...
- Page 2 Note The information in this manual only applies to TONOPORT VI, firmware version 3.0. It does not apply to earlier firmware versions. Due to continuing product innovation, specifications in this manual are subject to change without notice. CASE is a trademark owned by GE Medical Systems Information Technologies GmbH, a General Electric Company going to market as GE Healthcare.
-
Page 3: Table Of Contents
Contents Application, Safety Information Controls and Indicators Setup Application Data Output Error Codes Software Installation Cleaning, Maintenance, Disposal Technical Specifications Order Information Appendix - Electromagnetic Compatibility (EMC) 2001589-312 Revision B TONOPORT VI... - Page 4 Revision History Revision History This manual is subject to the GE Healthcare change order service. The revision code, a letter that follows the document part number, changes with every update of the manual. Part No./Revision Date Comment 2001589-312 Revision A...
- Page 5 General Information General Information The product TONOPORT VI bears the CE marking only once. Therefore, carefully read the manual CE 0482 (notified body MEDCERT GmbH) since once in its entirety. 2017 indicating its compliance with the provisions of The symbol...
- Page 6 Sachsendamm 6 10829 Berlin, Germany Germany Tel. +49 30 235 07 00 Fax +49 30 213 85 42 GE Medical Systems Information Technologies, Inc. 9900 Innovation Drive Wauwatosa, WI 53226 USA The country of manufacture is indicated on the device la- bel.
-
Page 7: Application, Safety Information
The blood pressure is TONOPORT VI can record up to 400 blood pressure determined either during deflation of the cuff (deflation measurements at selectable intervals and save the results. - Page 8 1.2 Functional Description The inflation measurement method is a novel method based on the "Inflation Measurement Technology (IMT)" The TONOPORT VI monitor accommodates the blood developed by PAR Medizintechnik. With this innovative pressure measuring system and a microprocessor for technique, the cuff is inflated to a pressure just above the system control and data processing.
- Page 9 • disconnection of items • update or upgrade of equipment – TONOPORT VI may be connected to CASE or to a PC with the CardioSoft program. While connected to any of these devices, TONOPORT VI must be disconnected from the patient.
- Page 10 Warning Caution Risk to Persons— Equipment damage, risk to persons— – Before cleaning, TONOPORT VI must be – Before connecting the battery charger to the power disconnected from other equipment (CASE, PC). line, check that the voltage ratings on the nameplate match those of your local power line.
-
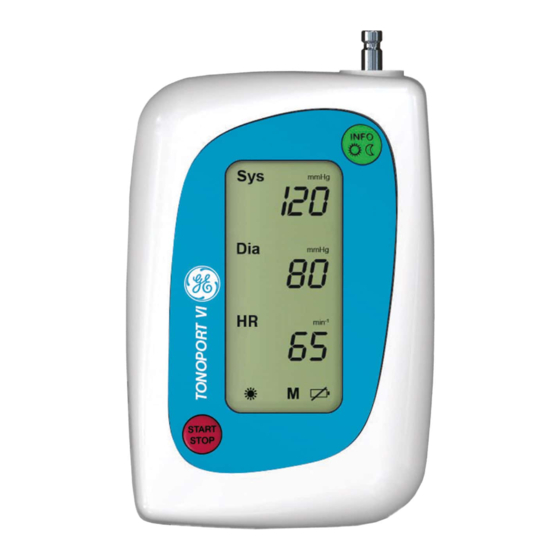
Page 11: Controls And Indicators
: push to start and stop a measure- START STOP ment and to confirm entries (Rechargeable) batteries Fig. 2-1 Controls and indicators of TONOPORT VI Lid covering battery compartment Nameplate Port for connection to PC (RS232) Port for connection to PC (USB) - Page 12 ES60601-1 (2005) + AMD 1 (2012), CAN/CSA-C22.2 No. 60601-1 (2014), IEC 60601-1-6 (2010, A1:2013), IEC 60601-1-11 (2015), IEC 80601-2-30 (2009, A1:2013) Caution: Federal law restricts this device to sale by or on the order of a physician. TONOPORT VI 2001589-312 Revision B...
-
Page 13: Setup
– Use only the original charger to recharge the NiMH batteries. China RoHS pollution control label – Do not attempt to recharge alkaline batteries. – If TONOPORT VI will not be used for one month RoHS Restriction of certain hazardous or more, remove the (rechargeable) batteries from substances. - Page 14 Turn on the BP monitor as follows: either by inserting the batteries or by briefly pressing If TONOPORT VI is powered by rechargeable batteries button. START (4 of them are shipped with the equipment), they should STOP ...
- Page 15 If the batteries are correctly inserted and the displayed battery symbols show no bars, the charger has identified a battery problem. The charging current will be cut off. Remove the batteries and discard, observing the applicable waste disposal regulations. 2001589-312 Revision B TONOPORT VI...
- Page 16 START STOP Performance Check When turned on, TONOPORT VI runs a self-test that includes all symbols and segments on the LCD (Fig. 3-4). Then it checks the batteries and indicates the remaining capacity. "A 100", for instance, means that the rechargeable batteries have a capacity of 100%, i.e., they...
- Page 17 Selecting the Measurement Method Before using TONOPORT VI on a patient clear the memory Briefly switch TONOPORT VI off and on again and wait for the time to be displayed. check date and time and adjust if required INFO ...
- Page 18 (10 p.m. to 7 a.m.) p.m.) Setting Time and Date every 15 minutes every 30 minutes Briefly switch TONOPORT VI off and on again and wait for the time to be displayed. every 20 minutes every 40 minutes INFO ...
-
Page 19: Application
INDEX label when the cuff is closed. The end of the cuff must be situated within this range when the cuff is closed. Latex-free blood pressure cuff. Single-use device. CE marking, cuff fulfills EU directives. 2001589-312 Revision B TONOPORT VI... - Page 20 – the cuff and the TONOPORT VI are used inside – By watching the limb it is necessary to check that the ambient conditions for operation and inside operation of the TONOPORT VI does not result in the measuring range (see chapter "Technical...
- Page 21 Performing a Trial Measurement Single-use cuffs are connected to the TONOPORT VI Turn on TONOPORT VI and place it in the wearable device by inserting the TONOPORT BP Single-Use Cuff pouch. There is an aperture in the pouch to accommo- Adapter between the device and the tube of the single-use date the cuff connection tube.
- Page 22 (see page 36). readings and to keep the cuff inflation time as short as possible – to place TONOPORT VI with the wearable pouch on Absolute contraindications: the night stand while in bed – how to switch the device manually from the day to the The application of the cuff is prohibited on an arm with night phase (refer to section "Toggling Between Day...
- Page 23 Toggling Between Day and Night Phase Measurement In the three measurement protocols, the day phase lasts These are the buttons on TONOPORT VI used during an from 7 a.m. to 10 p.m. and the night phase from 10 p.m. ambulatory blood pressure measurement: to 7 a.m.
-
Page 24: Data Output
TONOPORT VI is used (b, Fig. 5-1) – via cable 2001589-011 if the serial port of TONOPORT VI is used (a, Fig. 5-1) Turn on TONOPORT VI and wait for the time to be displayed. For more information about data output, please refer to the Operator Manual of CASE, CardioSoft. -
Page 25: Error Codes
6 mmHg/s. 40 mmHg and no diastolic pressure could be identified (TONOPORT VI does not measure For inflation measurement method: diastolic pressures below 40 mmHg). This error message will not be displayed because... -
Page 26: Software Installation
PC. Checking the Port USB port check only: For a check of the USB port, turn on TONOPORT VI and connect its USB port to the PC. Start the Device Manager of the operating system. Double-click Ports (COM and LPT) to view all ports. -
Page 27: Cleaning, Maintenance, Disposal
These checks shall be carried out by GE Healthcare or authorized companies. Caution Equipment Damage— The checks can be carried out by GE Healthcare within Do not disinfect the device surface with phenol- the framework of a service agreement; please contact GE based disinfectants or peroxide compounds. - Page 28 INFO Push four times: the display indicates "H 4". The checks can be carried out by GE Healthcare within Push : the display indicates an internal value START the framework of a service agreement; please contact GE STOP that must be between 25 and 100.
-
Page 29: Technical Specifications
– 2 AA size rechargeable NiMH batteries, 1.2 V, – IP20: TONOPORT VI >1500 mAh or – IP02: wearable pouch of the TONOPORT VI – 2 AA size alkaline batteries – IP22: TONOPORT VI in wearable pouch Battery Charge Time Expected Service Life –... -
Page 30: 10 Order Information
TONOPORT VI Ambulatory Blood Pressure System TONOPORT VI recording unit 2001589-014 Rechargeable NiMH battery (device Connection cable TONOPORT VI to PC (USB) requires 2) Connection cable TONOPORT VI to PC (RS232) 2001589-215 BP wearable pouch TONOPORT VI ... -
Page 31: Appendix - Electromagnetic Compatibility (Emc)
Guidance and Manufacturer’s Declaration—Electromagnetic Emissions TONOPORT VI is intended for use in the electromagnetic environment specified below. It is the responsibility of the customer or user to ensure that TONOPORT VI is used in such an environment. Emissions Test Compliance Electromagnetic Environment—Guidance... - Page 32 Electromagnetic Compatibility (EMC) Guidance and Manufacturer’s Declaration—Electromagnetic Immunity TONOPORT VI is intended for use in the electromagnetic environment specified below. It is the responsibility of the customer or user to ensure that TONOPORT VI is used in such an environment.
- Page 33 Electromagnetic Compatibility (EMC) Guidance and Manufacturer’s Declaration—Electromagnetic Immunity TONOPORT VI is intended for use in the electromagnetic environment specified below. It is the responsibility of the customer or user to ensure that TONOPORT VI is used in such an environment.
- Page 34 Recommended separation distances between portable and mobile RF communications equipment and TONOPORT VI TONOPORT VI is intended for use in the electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of TONOPORT VI can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and TONOPORT VI as recommended below, according to the maximum output power of the communications equipment.
- Page 35 Note Any supplied accessories that would not affect electromagnetic compatibility (EMC) are not included. 2001589-011 Connection cable TONOPORT VI to PC (RS232), length of 1.2 meters 2001589-040 Connection cable TONOPORT VI to PC (USB), length of 1.5 meters 2001589-312 Revision B...
- Page 36 Place the TONOPORT VI with the wearable pouch on your nightstand while you are sleeping. You are allowed to change the day phase and the night phase manually, if you go to bed before 10 pm or get up before 7 am. To change the...
- Page 37 Functional description 8 Technical inspections of the measuring system 28 General Information 5 Technical safety inspections 27 Technical specifications 29 Time, set 18 Toggling between night and day phases 23 Indicators 11 Trial measurement 21 2001589-312 Revision B TONOPORT VI...
- Page 38 Index USB driver installation 26 Warning 5 Weight 29 TONOPORT VI 2001589-312 Revision B...
- Page 40 PAR Medizintechnik GmbH & Co. KG Sachsendamm 6 10829 Berlin Germany Tel: +49 30 2350700 Fax: +49 30 2138542 GE Medical Systems Information Technologies, Inc. 9900 Innovation Drive Wauwatosa, WI 53226 USA www.gehealthcare.com...