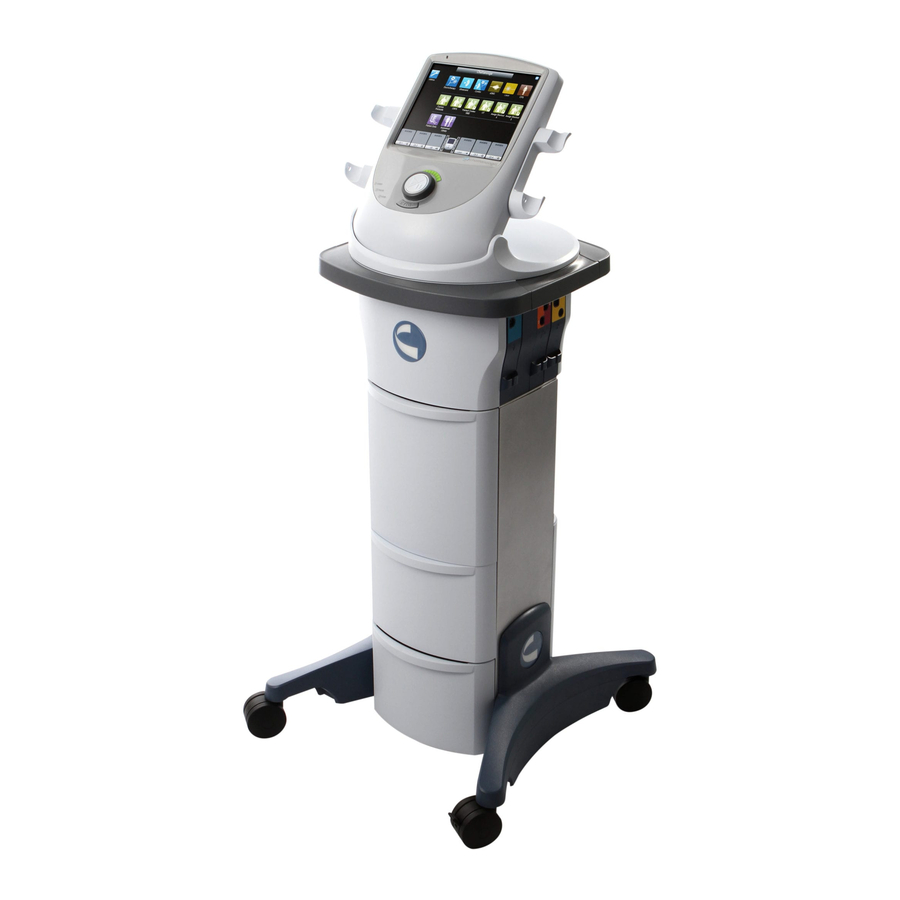
Summary of Contents for Chattanooga Vectra Neo
-
Page 1: User Manual
Vectra® Neo Clinical Therapy System User Manual Operator and Installation Instructions Rx Only... -
Page 3: Table Of Contents
Vectra® Neo Clinical Therapy System TABLE OF CONTENTS INTRODUCTION ....... . 4 SYSTEM SPECIFICATIONS . - Page 4 Vectra® Neo Clinical Therapy System APPENDIX 1 ........71 OVERVIEW OF LASER THERAPY .
-
Page 6: Introduction
INTRODUCTION Vectra® Neo Clinical Therapy System FOREWORD PRECAUTIONARY INSTRUCTIONS This manual is intended for users of Vectra® Neo Clinical The precautionary instructions found in this section and Therapy System. It contains general information on throughout this manual are indicated by specifi c symbols. operation, precautionary practices, and maintenance. -
Page 7: General Terminology
INTRODUCTION Vectra® Neo Clinical Therapy System GENERAL TERMINOLOGY The following are defi nitions for the terminology used throughout this manual. Study these terms to become familiar with them for ease of system operation and control functionality of the Vectra® Neo Clinical Therapy System. SYSTEM SOFTWARE SYMBOLS Back Arrow Stim... -
Page 8: Description Of Device Markings
INTRODUCTION Vectra® Neo Clinical Therapy System DESCRIPTION OF DEVICE MARKINGS The markings on the unit are assurance of its conformity to the highest applicable standards of medical equipment safety and electromagnetic compatibility. One or more of the following markings may appear on the device: Refer to Instructional Manual Booklet . -
Page 9: Indications For Use
INDICATIONS FOR USE Vectra® Neo Clinical Therapy System ELECTROTHERAPY INDICATIONS Indications • Do not use powered muscle stimulators or TENS waveforms on patients with cardiac demand For VMS (Pulsed Mode, Burst Mode, or FR Mode), pacemakers. Russian, Monophasic Hi-Volt (NMES) & Interferential, and •... -
Page 10: Semg & Stim Indications
INDICATIONS FOR USE Vectra® Neo Clinical Therapy System SEMG & STIM INDICATIONS Indications • Do not use Vectra® Neo Clinical Therapy System on patients with body worn electro mechanical medical For EMG triggered Stim: devices, i.e. insulin pump. • Do not use this system in an MRI or CT environment. •... -
Page 11: Ultrasound Indications
INDICATIONS FOR USE Vectra® Neo Clinical Therapy System ULTRASOUND INDICATIONS Indications • Do not use Vectra® Neo Clinical Therapy System on patients with body worn electro mechanical medical Application of therapeutic deep heat for the treatment of devices, i.e. insulin pump. selected sub-chronic and chronic medical conditions such •... -
Page 12: Laser Indications
INDICATIONS FOR USE Vectra® Neo Clinical Therapy System LASER INDICATIONS Indications • Do not use Vectra® Neo Clinical Therapy System on patients with body worn electro mechanical medical To provide topical heating for the following: devices, i.e. insulin pump. • Do not use this system in an MRI or CT environment. •... -
Page 13: Device Description
DEVICE DESCRIPTION Vectra® Neo Clinical Therapy System PRODUCT DESCRIPTION The Vectra® Neo Clinical Therapy System is a modular system used with or without an optional Cart, allowing for the inclusion of Channel 1/2 Electrotherapy module with or without sEMG, Channel 3/4 Electrotherapy module, Laser module, and Ultrasound module. -
Page 14: Operator Interface
DEVICE DESCRIPTION Vectra® Neo Clinical Therapy System OPERATOR INTERFACE The Vectra® Neo Clinical Therapy System Operator Front Controls Interface contains all the functions and controls necessary for operator access to all operator utilities, modalities, and parameters for modifi cation and system set up. 1. -
Page 15: General Warnings And Precautions
GENERAL WARNINGS AND PRECAUTIONS Vectra® Neo Clinical Therapy System CAUTION CAUTION • Read, understand, and practice the precautionary and operating • Failure to use and maintain the Vectra® Neo Clinical Therapy System, instructions. Know the limitations and hazards associated with using its modules, and its accessories in accordance with the instructions any electrical stimulation, Laser device, or ultrasound device. -
Page 16: Warning Notices
GENERAL WARNINGS AND PRECAUTIONS Vectra® Neo Clinical Therapy System WARNING WARNING • Do not treat through clothing. • U.S.A. Federal Law restricts these devices to sale by, or on the order of, a physician or licensed practitioner. This device should be used only •... - Page 17 GENERAL WARNINGS AND PRECAUTIONS Vectra® Neo Clinical Therapy System WARNING WARNING • The Vectra® Neo Clinical Therapy System may be susceptible to Electro- • Output current density is related to electrode size. Improper Static Discharge (ESD) at greater than ±4 kV when first grasping either application may result in patient injury.
-
Page 18: Danger Notices
Laser energy and the direction of the beam of light). When the unit is on, not all wavelengths are visible to the naked eye. Therefore, when performing any operational or functional check, always wear Chattanooga laser protective eyewear. • The solvents of adhesives and flammable solutions used for cleaning and disinfecting should be allowed to evaporate before the unit is used. -
Page 19: Detail Device Description
DETAIL DEVICE DESCRIPTION Vectra® Neo Clinical Therapy System COMPONENTS Modules Throughout these instructions the terms “left” and “right” referring to the machine sides are from the perspective of • Stimulation Channel 1/2 a user standing in front of the unit. •... -
Page 20: Module Slots
DETAIL DEVICE DESCRIPTION Vectra® Neo Clinical Therapy System MODULE SLOTS MODULE KIT CONTENTS 1. Laser Electrotherapy Module Channels 1/2 – PN 70000 • Stimulation module 2. Stimulation (1 & 2) / Stimulation (1 & 2) + sEMG • Lead wires •... -
Page 21: Ultrasound Applicator
DETAIL DEVICE DESCRIPTION Vectra® Neo Clinical Therapy System ULTRASOUND APPLICATOR 1. Applicator Head The component of the applicator that makes contact with the patient during Ultrasound or Combination therapy. 2. Applicator The assembly that connects to the system and incorporates the Applicator. 3. -
Page 22: Patient Remote/Laser Interrupt Switch
DETAIL DEVICE DESCRIPTION Vectra® Neo Clinical Therapy System PATIENT REMOTE/LASER INTERRUPT SWITCH The Vectra® Neo Patient Remote/Laser Interrupt Switch buttons are described in the table below. By default, the remote is not assigned to any treatment. When assigned, the buttons function as follows: Increase Intensity (1) Decrease Intensity (2) STOP... -
Page 23: Setup Instructions
SETUP INSTRUCTIONS Vectra® Neo Clinical Therapy System HEAD TO CART ASSEMBLY The optional Therapy System Cart, PN 70001, is designed 3. Place the Neo Head on the cart facing toward the for use with the Vectra® Neo Clinical Therapy System only drawers. -
Page 24: Neo Leg To Cart Assembly/Adjustment
SETUP INSTRUCTIONS Vectra® Neo Clinical Therapy System NEO LEG TO CART ASSEMBLY/ADJUSTMENT The Neo Cart is shipped without the legs attached. 2. There are two Cart height adjustments. Standard shown on the left and lowered, shown on the right. Tools Required: For initial installation, determine the desired height. -
Page 25: Module Installation
SETUP INSTRUCTIONS Vectra® Neo Clinical Therapy System MODULE INSTALLATION All modules are installed from the left side (when facing 3. Insert a standard slotted screwdriver (not provided) the screen) of the Neo Head unit and are each installed into the top slot, pressing down with slight pressure. in the same manner. -
Page 26: Module-Specific Information
SETUP INSTRUCTIONS Vectra® Neo Clinical Therapy System MODULE INSTALLATION (CONTINUED) MODULE-SPECIFIC INFORMATION 6. Secure the module with a screw provided at the Ultrasound Cable Insertion bottom as shown (using channel 3/4 as example). Shown below is the Ultrasound Cable Insertion location. 7. -
Page 27: Patient Remote/Laser Interrupt Switch
SETUP INSTRUCTIONS Vectra® Neo Clinical Therapy System PATIENT REMOTE/LASER INTERRUPT SWITCH INSTALLATION To operate the Patient Remote/Laser Interrupt Switch, 2. Press the Remote ON/OFF toggle icon to assign or plug the remote into the device on the Rear Access Panel unassign the remote to the selected treatment. -
Page 28: Installing The Laser Interlock (Door Interrupt Switch)
SETUP INSTRUCTIONS Vectra® Neo Clinical Therapy System INSTALLING THE LASER INTERLOCK (DOOR INTERRUPT SWITCH) Diagram for Therapy Room with One Door The Laser Interlock is an optional safety device designed to interrupt Laser therapy if the door to the therapy room is opened. -
Page 29: Therapy System Start-Up
SETUP INSTRUCTIONS Vectra® Neo Clinical Therapy System THERAPY SYSTEM START-UP Complete the following steps for initial setup of the 3. Select desired function on the Home screen (shown Vectra® Neo Clinical Therapy System: below). 1. Plug the Power cord into the back of device. Plug the other end of the cord into an electrical outlet. -
Page 30: System Specifications
SYSTEM SPECIFICATIONS Vectra® Neo Clinical Therapy System SYSTEM SPECIFICATIONS AND DIMENSIONS Width Depth Height Weight Module 11.12” (28.2448 cm) 6.34” (16.1036 cm) 1.43” (3.6322 cm) 1lb (0.453592 kg) Head @ 45 degree with Base (Tabletop) 15.89” (40.3606 cm) 15.89” (40.3606 cm) 22.05”... -
Page 31: Ultrasound Specifications
SYSTEM SPECIFICATIONS Vectra® Neo Clinical Therapy System ULTRASOUND SPECIFICATIONS ULTRASOUND SPATIAL PATTERN The following charts represent the distribution of the Frequency....... . . 1 MHz, ±5%; 3.3 Mhz, ±5% ultrasonic radiation fi eld and the orientation of the fi eld Duty Cycles . -
Page 32: System Specifications
SYSTEM SPECIFICATIONS Vectra® Neo Clinical Therapy System LASER SPECIFICATIONS Power Output Type ........Infrared Lamp (Laser) Laser Class. -
Page 33: Laser Applicator Technical Specifications
SYSTEM SPECIFICATIONS Vectra® Neo Clinical Therapy System LASER APPLICATOR TECHNICAL SPECIFICATIONS For all single diode and cluster laser and LED applicators, the expected increase in the measured quantities after manufacture added to the values measured at the time of manufacture is 20%. - Page 34 SYSTEM SPECIFICATIONS Vectra® Neo Clinical Therapy System LASER APPLICATOR TECHNICAL SPECIFICATIONS (CONTINUED) Output Power Nominal Ocular Divergence Divergence Diode Wavelength(s) Treatment Area Model # Description Power Density Hazard Distance Type (nm) (Spot Size) (cm (mW) (W/cm (NOHD-in meters) (rad) (rad) 9-Diode GaAIAs 670 nm (10mW) LED x4...
-
Page 35: Laser Protective Eyewear Specifications
SYSTEM SPECIFICATIONS Vectra® Neo Clinical Therapy System LASER PROTECTIVE EYEWEAR SPECIFICATIONS The graph below illustrates optical density in relation to wavelength. Each unit is shipped with laser protective eyewear that is L3 rated and approved, as well as EN207 compliant. Wavelength Useful Range Optical Density 5+. -
Page 36: Laser Labels
SYSTEM SPECIFICATIONS Vectra® Neo Clinical Therapy System LASER LABELS This serial decal label is affi xed at the rear of the system. Complies with 21CFR 1040.10 21CFR 1040.11 IEC 60601-1 IEC 60601-1-2 IEC 60601-2-22 IEC 60601-2-57 IEC 62471 IEC 60825-1:2007 DJO, LLC 1430 Decision Street Vista, CA 92081... -
Page 37: Waveforms
SYSTEM SPECIFICATIONS Vectra® Neo Clinical Therapy System WAVEFORMS For additional information on Waveforms and output energy, refer to APPENDIX 3 on page 78. TENS- Asymmetrical Biphasic CC: Constant Current The Asymmetrical Biphasic waveform has a short pulse duration. It is capable CV: Constant Voltage of strong stimulation of the nerve fi bers in the skin as well as of muscle tissue. - Page 38 SYSTEM SPECIFICATIONS Vectra® Neo Clinical Therapy System WAVEFORMS (CONTINUED) VMS™ VMS™ FR VMS™ is a symmetrical biphasic waveform with a 100 μsec interphase interval. The VMS™ FR version of the VMS ™ waveform is a physiologically based channel Because the pulse is relatively short, the waveform has a low skin load, interaction in which one channel stimulates the agonist and the other the making it suitable for applications requiring high intensities, such as in muscle antagonist of the muscle group that is being exercised.
- Page 39 SYSTEM SPECIFICATIONS Vectra® Neo Clinical Therapy System WAVEFORMS (CONTINUED) DC (Direct Current) High Voltage Pulsed Current (HVPC) DC Current is a direct current fl owing in one direction only. The current can be The High Voltage Pulsed Current (HVPC) has a very brief pulse duration continuous or interrupted.
-
Page 40: Patient Preparation
PATIENT PREPARATION Vectra® Neo Clinical Therapy System ELECTRODE PLACEMENT ELECTROTHERAPY PATIENT PREPARATION • Examine the skin for any wounds and clean the skin. DURA-STICK® Electrode Instructions • Apply the electrodes to the treatment area. Connecting Lead Wires • Ensure the electrodes are applied securely to the skin. •... -
Page 41: Semg & Stim Patient Preparation
PATIENT PREPARATION Vectra® Neo Clinical Therapy System SEMG & STIM PATIENT PREPARATION 2. Select the Customize icon. Install DURA-STICK® Electrodes 3. Select the Electrode Placement icon to view electrode 1. Connect a DURA-STICK® 2 in (5 cm) disposable placement according to body area. electrode to each lead. - Page 42 PATIENT PREPARATION Vectra® Neo Clinical Therapy System sEMG & STIM PATIENT PREPARATION (CONTINUED) 8. Thoroughly cleanse the skin treating area. 3. Review the specific Electrode Placement graphic for positioning of the Reference (green lead) electrode. NOTE: Thorough and proper cleaning of the treatment area to remove any topical medication NOTE: The electrodes may be placed for specific, and cream film as well as loose skin particles from...
-
Page 43: Laser Patient Preparation
PATIENT PREPARATION Vectra® Neo Clinical Therapy System LASER PATIENT PREPARATION ULTRASOUND PATIENT PREPARATION Preparing the Patient’s Skin for Laser Therapy 1. Examine the skin for any wounds and clean the skin. 2. View the Applicator recommendation in the Before applying Laser therapy to the patient, you must treatment. -
Page 44: Device User Interface
DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System SCREEN DESCRIPTION • Setup: Indicates a treatment for the channel is Each screen contains the following areas: currently being set up but treatment has not yet Title Bar begun • Running: Indicates a treatment for the channel is Located at the top of each screen and lists the current currently running screen and previous screens back to the Home screen. -
Page 45: Home Screen
DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System HOME SCREEN The Vectra® Neo Clinical Therapy System Home screen provides access to all of the system modalities and functions. The Home screen has the following information: 1. Utilities Modality Icons: 2. Electrotherapy 3. -
Page 46: Utilities And Options
DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System UTILITIES AND OPTIONS The Utilities icon on the Home screen off ers users the opportunity to set the following preferences:: 1. Print Screen 4. US Coupling Select the Printer icon in the top right corner to Select the US Coupling icon to set the functionality of capture screen shots of current screen. - Page 47 DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System UTILITIES AND OPTIONS (CONTINUED) 5. US Head Warming 11. Import Protocols from USB Flash Drive Select the US Head Warming icon to turn on or off Select the Import Protocols from USB Flash Drive icon the ultrasound warming feature during ultrasound to import all protocols from a valid USB flash drive.
-
Page 48: Treatment Screens
DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System TREATMENT SCREENS The Vectra® Neo Clinical Therapy System Treatment screens for Electrotherapy and Ultrasound include the following information: 1. Modality Description Icon 8. Therapy Information Window Press the Modality Description Icon to view the text View selected Therapy information such as Waveform, explaining the rationale for the modality associated Electrode Size, CC/CV, Carrier Frequency, Cycle Time,... -
Page 49: Cps (Clinical Protocol Setup)
DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System CPS (CLINICAL PROTOCOL SETUP) 9. Overwrite a previously saved protocol by using the Up The Vectra® Neo Clinical Therapy System has a Clinical and Down arrows or Previous Page/Next Page icons to Protocol Setup (CPS) icon that is a series of protocol select the protocol to overwrite. -
Page 50: Electrotherapy Operation
DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System ELECTROTHERAPY OPERATION All waveforms in the Vectra® Neo Clinical Therapy System • To view information explaining the waveform, press are set up and edited in the same basic fashion. The the Modality Description icon. Press the Up and Vectra®... - Page 51 DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System ELECTROTHERAPY OPERATION (CONTINUED) 5. If desired, connect optional Remote Control to device. 6. Use the Intensity dial to set therapy intensity: • Clockwise - Increases Intensity • Counterclockwise - Decreases Intensity 7. Press the Start button to begin therapy, the Pause button to pause treatment, or the Stop button to terminate the treatment.
-
Page 52: Sequencing Operation
DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System SEQUENCING OPERATION The Vectra® Neo Clinical Therapy System off ers sequencing 4. Make any desired changes to the existing sequences for special electrotherapy waveform treatment purposes by using the Up and Down arrows to find the desired and stores these protocols in the system memory for sequence and press the Edit icon. -
Page 53: Ultrasound Operation
DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System ULTRASOUND OPERATION The Vectra® Neo Clinical Therapy System Ultrasound Complete the following steps to begin Ultrasound modality allows the user to select specifi c Applicator treatment: recommendations and edit treatment parameters for 1. - Page 54 DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System ULTRASOUND OPERATION (CONTINUED) 3. Press the Start button to begin therapy, the Pause button to pause treatment, or the Stop button to terminate the treatment. 4. When treatment has completed, the Treatment Summary screen will appear with the following options: •...
-
Page 55: Combination Operation
Vectra® Neo Clinical Therapy System COMBINATION OPERATION The Vectra® Neo Clinical Therapy System Combination 5. Select the ultrasound combination therapy desired, modality allows the user to select and use Ultrasound and press the corresponding icon. The Treatment therapy in combination with electrical muscle stimulation. Review screen shown below will appear: Combination therapy utilizes the Ultrasound modality in conjunction with High Voltage Pulsed Current (HVPC),... - Page 56 DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System COMBINATION OPERATION (CONTINUED) • To customize settings shown in the list boxes, press 6. Press the Start button to begin therapy, the Pause the Customize icon located in the list box, and the button to pause treatment, or the Stop button to screen shown below will appear.
-
Page 57: Semg Operation
DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System sEMG OPERATION The Vectra® Neo Clinical Therapy System sEMG + Stim 4. Select the prescribed channel icon (see previous modality utilizes sEMG biofeedback activity coupled with for the available choices). The treatment screen will triggered electrical muscle stimulation using selected appear (the screen shown below illustrates the sEMG electrotherapy waveforms for the maximum benefi t in... -
Page 58: Laser Operation
DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System sEMG OPERATION (CONTINUED) LASER OPERATION 6. The following options are available under the Before applying Laser therapy to the patient, you must Customize Treatment screen and accessed by pressing fi rst prepare the patient’s skin as described in the the appropriate icon: PATIENT PREPARATION section on page 38. - Page 59 DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System LASER OPERATION (CONTINUED) Complete the following steps to begin Laser therapy • To customize settings shown in the list boxes, press treatment: the Customize icon located in the list box, and the screen shown below will appear.
-
Page 60: Saving To Usb Flash Drive/Patient Data
DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System SAVING TO USB FLASH DRIVE/PATIENT DATA Patient treatment data can be saved to the USB fl ash drive 5. Choose one of the following options from the for viewing/printing on a PC, as well as for retrieving for Treatment Summary screen: later use on the unit or multiple units. -
Page 61: Custom Protocols
DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System CUSTOM PROTOCOLS A new custom protocol may be saved at either the 3. After saving the protocol, you will return to the Treatment Edit or Treatment Complete screen. The Vectra® Treatment Summary screen. Complete one of the Neo Clinical Therapy System will allow a maximum of 200 following actions listed on the Treatment Summary custom protocols to be defi ned, and the Home screen will... - Page 62 DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System CUSTOM PROTOCOLS (CONTINUED) Complete the following steps to delete a previously saved Complete the following steps to assign a Home screen protocol: shortcut for a customized protocol: 1. From the Home screen, press the Custom Protocols 1.
- Page 63 DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System CUSTOM PROTOCOLS (CONTINUED) Complete the following steps to deassign a Home screen Complete the following steps to view and access patient shortcut for a customized protocol: data: 1. From the Home screen, press and hold the shortcut 1.
-
Page 64: Anatomical Library
DEVICE USER INTERFACE Vectra® Neo Clinical Therapy System ANATOMICAL LIBRARY The Vectra® Neo Clinical Therapy System contains a 4. View the selected image and, if desired, print the unique Anatomical Library designed to aid the operator selected image by pressing the printer icon: in visually understanding and locating specifi c muscle groups and commonly found problems associated with pathological conditions, as well as providing an... -
Page 65: Troubleshooting
TROUBLESHOOTING Vectra® Neo Clinical Therapy System TROUBLESHOOTING CODES The Vectra® Neo Clinical Therapy System incorporates information, warning, and error messages to inform the user of problems or potential problems with the system, modality, or accessories. The numbering sequence is: 100-level messages are general use information messages, 200-level messages are warnings, and 300-level messages are errors. - Page 66 TROUBLESHOOTING Vectra® Neo Clinical Therapy System TROUBLESHOOTING CODES (CONTINUED) Code Type Message Probable Cause Possible Remedies Number 1. If a Stim module is installed in the unit, ensure module is securely inserted into the Attempting to select an electrotherapy treatment but no Stim unit.
- Page 67 TROUBLESHOOTING Vectra® Neo Clinical Therapy System TROUBLESHOOTING CODES (CONTINUED) Code Type Message Probable Cause Possible Remedies Number Any 200-level Warning Immediately stop all use of the system and contact the dealer or DJO for service. Warnings in these categories indicate an internal problem code not listed with the system that must be tested and corrected by DJO or a Trained Technician before any further operation or use of the system.
-
Page 68: Accessories
ACCESSORIES Vectra® Neo Clinical Therapy System REPLACEMENT ACCESSORIES The following provides users of the Vectra® Neo Clinical Therapy System the necessary information to order replacement accessories used with the system. This list of replacement accessories is designed for use with the Vectra® Neo Clinical Therapy System. - Page 69 ACCESSORIES Vectra® Neo Clinical Therapy System REPLACEMENT ACCESSORIES (CONTINUED) LASER APPLICATORS AND EYEWEAR ACCESSORIES Model Number Description 27840 Single 850nm Laser 100mW 27804 Single 850nm Laser 150mW 27841 Single 850nm Laser 200mW 27803 Single 850nm Laser 40mW 27805 Single 820nm Laser 300mW 27810 9-Diode cluster: 5-50mW laser diodes + 4 LED’s 27811...
-
Page 70: Maintenance
MAINTENANCE Vectra® Neo Clinical Therapy System CLEANING THE VECTRA® NEO CLINICAL FUSE INFORMATION THERAPY SYSTEM With the system disconnected from the power source, clean the system with a clean, lint-free cloth moistened 3.15A, 250V AC with water and mild antibacterial soap. If a more sterile cleaning is needed, use a cloth moistened with an antimicrobial cleaner. -
Page 71: Service And Warranty
SERVICE AND WARRANTY Vectra® Neo Clinical Therapy System WARRANTY REPAIR/OUT OF WARRANTY REPAIR Service When the Vectra® Neo Clinical Therapy System or any of the accessory modules require service, contact the selling dealer or DJO Service Department. All Therapy System and accessory modules returned to the factory for service must include the following: 1. -
Page 72: Warranty
SERVICE AND WARRANTY Vectra® Neo Clinical Therapy System WARRANTY COMPANY SHALL NOT BE LIABLE IN ANY EVENT FOR DJO, LLC (“Company”) warrants that the Vectra® Neo INCIDENTAL OR CONSEQUENTIAL DAMAGES. Clinical Therapy System, Channel 1/2 Electrotherapy Module, Channel 1/2 Electrotherapy+EMG Module, Some states do not allow the exclusion or limitation Channel 3/4 Electrotherapy Module, Laser Module, and of incidental or consequential damages, so the above... -
Page 73: Overview Of Laser Therapy
APPENDIX 1 Vectra® Neo Clinical Therapy System OVERVIEW OF LASER THERAPY TREATMENT TIPS Laser therapy results in energy absorbed into the patient’s Contact tissue from light, triggering biological changes at a To obtain the most eff ective results, the applicator should cellular level, resulting in the following: be in contact with the patient’s skin. -
Page 74: Common Terms
APPENDIX 1 Vectra® Neo Clinical Therapy System COMMON TERMS Treatment Time – Measured in seconds, it is the Applicator – Hand held assembly that delivers Laser suggested time per laser point that therapy is given energy and includes laser head, diode, and related electronics Wavelength –... -
Page 75: Electromagnetic Compatibility (Emc)
APPENDIX 2 Vectra® Neo Clinical Therapy System ELECTROMAGNETIC COMPATIBILITY (EMC) The Vectra® Neo Clinical Therapy System has been Caution: tested and found to comply with the electromagnetic Medical electrical equipment requires special precautions compatibility (EMC) limits for medical devices to regarding EMC and must be installed and operated IEC 60601-1-2. -
Page 76: Tables
APPENDIX 2 Vectra® Neo Clinical Therapy System ELECTROMAGNETIC COMPATIBILITY (EMC) TABLES Guidance and Manufacturer’s Declaration - Electromagnetic Emissions The Vectra® Neo Clinical Therapy System is intended for use in the electromagnetic environment specifi ed below. The customer or the user of the Vectra® Neo Clinical Therapy System should assure that it is used in such an environment. Emissions Tests Compliance Electromagnetic Environment - Guidance... - Page 77 61000-4-2 ESD-precautionary measures are taken. The Vectra Neo Clinical Therapy System may be susceptible to Electro-Static Discharge (ESD) at greater than ±4 kV when fi rst grasping either the Ultrasound or Laser applicator. In the event...
- Page 78 APPENDIX 2 Vectra® Neo Clinical Therapy System ELECTROMAGNETIC COMPATIBILITY (EMC) TABLES (CONTINUED) Guidance and Manufacturer’s Declaration - Electromagnetic Immunity The Vectra® Neo Clinical Therapy System is intended for use in the electromagnetic environment specifi ed below. The customer or the user of the Vectra® Neo Clinical Therapy System should assure that it is used in such an environment. IEC 60601 Immunity Test Compliance Level...
- Page 79 APPENDIX 2 Vectra® Neo Clinical Therapy System ELECTROMAGNETIC COMPATIBILITY (EMC) TABLES (CONTINUED) Recommended separation distances between portable and mobile RF communications equipment and the Vectra® Neo Clinical Therapy System The Vectra® Neo Clinical Therapy System is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled.
-
Page 80: Calculations For Output Current
APPENDIX 3 Vectra® Neo Clinical Therapy System CALCULATIONS FOR OUTPUT CURRENT The Vectra® Neo Clinical Therapy System controls total output current and output energy to comply with IEC 60601-2- 10. The System also controls total output current density for the selected electrode to comply with FDA requirements. The Vectra®... - Page 81 APPENDIX 3 Vectra® Neo Clinical Therapy System CALCULATIONS FOR OUTPUT CURRENT (CONTINUED) IEC current Intensity [I] Phase Duration Output current equation Frequency [F] Number of Waveform limit (in mA (CC), in Duty Cycle [D] in mA] (in Hz) Pulse [N] (in mA) V (CV)) (in uSec)
-
Page 82: Maximum Current Output Calculations Based On 250 Mw/Cm Power Density Limit
APPENDIX 3 Vectra® Neo Clinical Therapy System MAXIMUM CURRENT OUTPUT CALCULATIONS BASED ON 250 MW/CM POWER DENSITY LIMIT Maximum RMS Maximum RMS Electrode Part Dimensions (inch) Maximum Power Area (cm Current Based on Current Allowed by Number (shape) Density (mW/cm Power Density (mA) System (mA) 42045... -
Page 83: Current Density Calculations For Djo Electrodes
APPENDIX 3 Vectra® Neo Clinical Therapy System CURRENT DENSITY CALCULATIONS FOR DJO ELECTRODES... -
Page 84: Electrode Size Selection
APPENDIX 3 Vectra® Neo Clinical Therapy System ELECTRODE SIZE SELECTION The Vectra® Neo Clinical Therapy System allows the user to select an electrode size from three diff erent options, which allows the System to more accurately control the output current. Please refer to following table for the selection guide related to diff erent DJO electrode part numbers. - Page 86 DJO, LLC A DJO Global Company 1430 Decision Street Vista, CA 92081-8553 USA T: 1-800-592-7329 USA F: 1-760-734-5608 DJOGlobal.com © 2014 DJO, LLC. All rights reserved. Vectra® Neo Clinical Therapy System Manual 13-7646 Rev E 11/2014...

















Need help?
Do you have a question about the Vectra Neo and is the answer not in the manual?
Questions and answers