RhythMedix RhythmStar Operator's Manual
Mobile cardiac monitor
Hide thumbs
Also See for RhythmStar:
- Quick start manual (2 pages) ,
- Quick start manual (2 pages) ,
- Setup manual (28 pages)
Table of Contents
Advertisement
Advertisement
Table of Contents

Summary of Contents for RhythMedix RhythmStar
- Page 1 Mobile Cardiac Monitor Operator Manual Revison 0.8.6 Document No. 01424...
-
Page 2: Table Of Contents
Table of Contents Description…………………………………………………………….. 3 Indications for Use…………………………………………………… Contraindications for Use…………………………………………… 4 Precautions……………………………………………………………. Parts…………………………………………………………………….. 6 5.1 RhythmStar Monitor……………………………………………….. 6 5.2 ECG Cable………………………………………………………… 5.3 Batteries……………………………………………………………. 8 5.4 Battery Charger……………………………………………………. 9 Electrode Application-Connecting the ECG…………………….. 6.1 Connecting the ECG cable to the patient……………………….. 9 6.1.1 2-Channel (3-lead) Electrode Placement…………………….. -
Page 3: Description
3rd party lead electrodes supplied to a patient by a physician or a monitoring enter. High quality FDA approved lead electrodes should be used. The RhythmStar system supports USB connectivity that can be used to send and receive data from/to RhythmStar and other devices. The server can deliver... -
Page 4: Indications For Use
2. Indications for Use The RhythmStar System is intended for use by patients who either have or are at risk of having cardiac disease and those that demonstrate intermittent symptoms indicative of cardiac disease and require cardiac monitoring on a continuing basis. -
Page 5: Precautions
should not be considered true representations of the actual pacemaker stimulus amplitude. To avoid unintended battery discharge, do not leave the battery in RhythmStar when it is not in use. To receive the best recording results, instruct patients to stay away from heavy ... -
Page 6: Parts
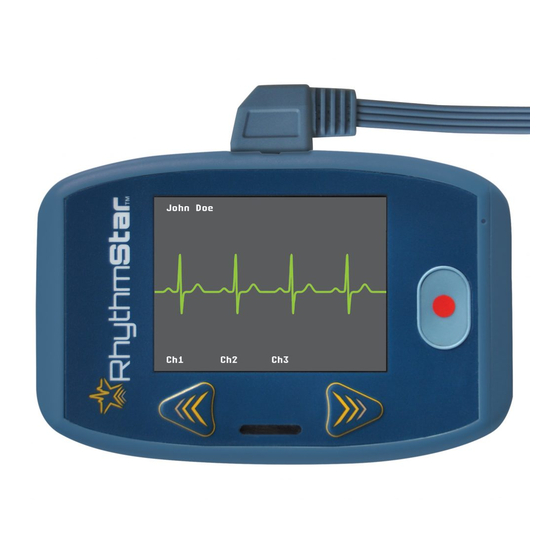
RhythmStar is shipped with the following components: 5.1 RhythmStar monitor The RhythmStar monitor is a small, lightweight, portable, battery powered device which is typically worn on a patient’s belt or waistband by placing the monitor in a “cell phone type pouch” (not supplied by RhythMedix). - Page 7 Monitor Components continued Letter Description Battery Compartment Serial Number Battery Door Back view of the RhythmStar Monitor (battery door removed, no battery inserted) D E F Revison 0.8.6 Document No. 01424 Page 7...
-
Page 8: Ecg Cable
5.2 ECG Cable The patient’s ECG signal is conducted using the patient ECG lead cable. RhythmStar will not work with any other cables than those supplied by the manufacturer. With RhythmStar, you can choose between a 3– or a 5-lead ECG cable. -
Page 9: Battery Charger
RhythmStar batteries must be charged with the included battery charger. 6. Electrode Application—Connecting the ECG NOTE: RhythmStar device is intended to be used with 3rd party lead electrodes supplied to a patient by a physician or a monitoring center. High quality FDA ap- proved lead electrodes should be used. -
Page 10: 2-Channel (3-Lead) Electrode Placement
STEP 1: Connect the patient ECG lead cable snap to the electrode. STEP 2: Remove the protective backing from the electrode. STEP 3: Apply the electrode to the patient’s skin. Apply each electrode to match Figures 1 or 2 in this manual or as instructed by the physician. ... -
Page 11: 3-Channel (5-Lead) Electrode Placement
6.1.2 5-lead configuration Five color-coded lead wires are used to create three channels of ECG recording. This is a typical electrode placement: Figure 2—5-Lead Electrode Placement Color Location White Upper-right portion of chest, 2 to 3 inches below collarbone Below left breast, over lower rib- cage Black Upper-left portion of chest, 2 to 3... -
Page 12: Connecting The Patient Ecg Lead Cable To The Monitor
7. Using the monitor 7.1 Powering on the monitor RhythmStar does not contain a separate On/Off power button. When you are ready to begin using RhythmStar, insert a charged battery into the monitor as pictured on the following page. Revison 0.8.6 Document No. - Page 13 Step 4: If the battery was successfully installed, after a few seconds, the screen should show the RhythmStar name and logo as pictured. RhythmStar is now powered and ready for use.
-
Page 14: Checking Ecg Signal Quality
7.2 Checking ECG signal quality To visualize the patient’s ECG signal on the RhythmStar’s LCD screen to inspect the quality of the ECG signal, follow the steps below: Step 1: Make sure the patient ECG lead cable is connected to RhythmStar and insert the battery. -
Page 15: Recording A Cardiac Event
The patient should be instructed to press the Record Button to start a recording when he or she experiences symptoms for which RhythmStar was prescribed. It is recommended that this feature be used by patients who can comprehend the instructions provided by the medical technician or physician and are capable of pressing the necessary buttons. -
Page 16: Entering Symptoms And Activity Level Associated With The Cardiac Event
7.4 Entering symptoms and activity level associated with the cardiac event After a recording is completed, RhythmStar will then prompt the patient to select the symptom or symptoms and the level of activity that he or she was feeling and doing at the time of pressing the Record Button. -
Page 17: Recharging The Battery
RhythmStar monitor, the other battery is being charged. We recommend replacing the battery being used in the RhythmStar monitor with a fully charged battery every 24 hours to avoid any power outage on the monitor while RhythmStar is monitoring the patient. -
Page 18: Maintenance And Service
8.2 Service If there is a problem with the monitor, review the Troubleshooting section for a listing of problems and solutions. If additional assistance is required, contact RhythMedix customer support via phone at (856) 282-1080. Be prepared to provide the following... -
Page 19: Troubleshooting
8.3 Troubleshooting Revison 0.8.6 Document No. 01424 Page 19... -
Page 20: Disposal Of Battery
8.4 Disposal of Battery Storage and Disposing of Lithium-Ion (Li-Ion) batteries instructions: The RhythmStar monitor and battery should be stored at room temperature in a dry area. Make sure that the battery is removed from the monitor while in storage. -
Page 21: Medical Device Symbols And Safety Signs
9. Medical Device Symbols and Safety Signs Revison 0.8.6 Document No. 01424 Page 21... -
Page 22: Specifications
10. Specifications Characteristics Test Conditions Min. Typical Max. Unit Physical Length Width Thickness Weight With battery Functional ECG Channels Cable selectable Accelerometer ±2g/±4g/±8g Sampling Rate Dynamically selectable 1.56 Variable Bits Resolution Memory Recording Time days Data Retention microSDHC flash media years Wireless Communication Technology... -
Page 23: Rhythmedix Limited Warranty
Except for the express warranties stated above, RhythMedix disclaims all warranties including implied warranties of merchantability and fitness. The stated express warranties are in lieu of all obligations of liabilities on the part of RhythMedix for damages, including but not limited to, special indirect or consequential, arising out of or in connection with the use or performance of RhythMedix products. -
Page 24: Wireless Compliance
FCC recommended limits. The RhythmStar contains a radio transmitter and receiver. It is designed and manufactured not to exceed the emission limits for exposure to radio frequency (RF) energy set by the Federal Com- munications Commission of the U.S. -
Page 25: Fcc Statement
Third-party belt-clips, holsters, and similar accessories used by this device should not contain any metallic components. Body-worn accessories that do not meet these requirements may not comply with RF exposure requirements and should be avoided. FCC statement · This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions: (1) This device may not cause harmful interference, and (2) this device must accept any interference received, including interference that may cause undesired operation. - Page 26 IC ID: 11948A-10002 Canadian Regulations Compliance Statement RhythmStar Complies with the Canadian RSS - 102 Issue 4: 2010 and IEC 62209-2: 2010 re- quirements. RhythmStar Conforme à la norme RSS - 102 Issue 4: 2010 et IEC 62209-2: 2010 reguirements. This device complies with RSS 210 of Industry Canada. This Class B device meets all the requirements of the Canadian interference-causing equipment regulations.
-
Page 27: Arrhythmia Detection Performance
14. Arrhythmia Detection Performance The RhythmStar incorporates a real-time embedded arrhythmia detection algorithm. The processing steps include signal bandpass and morphological filtering, estimation of the motion artifact, analysis of the slope, width and amplitude of the signal, decision making logic to determine location and length of the QRS complexes, atrial activity analysis and template matching for AF detection. -
Page 28: Accessories
15. Accessories The following accessories are available for use with the RhythmStar: Part Number: Description: PC-10003 RhythmStar two-channel patient ECG lead cable PC-10005 RhythmStar three-channel patient ECG lead cable BP-10006 RhythmStar Rechargeable Li-Ion battery ... -
Page 29: Appendix A-Handling Instructions For Lithium Ion Battery
Appendix A—Handling Instructions for Lithium Ion Battery Please read and follow the handling instructions for the battery before use. Improp- er use of the battery may cause heat, fire, explosion, damage or capacity deterio- ration of the battery. However, the manufacturer will not guarantee against any ac- cident caused by the usage which is not written here. - Page 30 Leaving it, it is may cause a rash on skin. If you have any question regarding the battery, contact RhythMedix. Read the instructions of your equipment regarding the battery installation and removal from the equipment so as not to mishandle and waste the battery.
- Page 31 (When charging the battery) DANGER Do not use any battery charger not specified by ( manufacturer's name ), also, follow the charge conditions specified by ( manufacturer's name ) If the battery is charged under other conditions ( a high temperature, a high voltage or current, or an altered charger ) not specified by ( manufacturer's name ), the battery may cause heat generation, explosion, or fire with abnormal chemical reactions.
- Page 32 RhythMedix, LLC. Horizon Corporate Center 5000 Atrium Way, Ste 1 Mt. Laurel, NJ 08054 Tel 856.282.1080 Fax 856.282.1333 www.rhythmedix.com Revison 0.8.6 Document No. 01424...


Need help?
Do you have a question about the RhythmStar and is the answer not in the manual?
Questions and answers
While the RhythmStar is being worn, should the green MONITORING bar be visible ? Or does the screen go blank until a notification that Is the Monitoring Center, the doctor/facility that provided the monitor to me? IF it is not the doctor, what number do I call?
Where do you get a plastic belt clip for the monitor.
what number to call to report an incident
The contact number to report an incident for RhythMedix RhythmStar is (856) 282-1080.
This answer is automatically generated
my Rythmn star monitor is blinking white
@Mary G Sullivan