Table of Contents
Advertisement
ATTENTION: RISK MANAGEMENT AND CHIEF MEDICAL OFFICER
QUALITY ASSURANCE AND
QUALITY ASSURANCE AND
QUALITY CONTROL REFERENCE GUIDE
QUALITY CONTROL REFERENCE GUIDE
FOR MULTI-PATIENT USE FACILITIES
FOR MULTI-PATIENT USE FACILITIES
Information in This Manual is Specific for Healthcare Providers
ATTENTION:
TRUEtest
Strips contain GDH-PQQ
™
enzyme. Please carefully review all critical
safety information and instructional
materials prior to performing patient
blood glucose testing.
www.homediagnostics.com
1-800-803-6025 or 1-954-677-4599.
© 2010 Home Diagnostics, Inc. TRUEresult and TRUEtest are trademarks of Home Diagnostics, Inc. E9SUN21 Rev. 3 • E9051-90
Advertisement
Table of Contents

Summary of Contents for McKesson TRUEresult
- Page 1 Please carefully review all critical safety information and instructional materials prior to performing patient blood glucose testing. www.homediagnostics.com 1-800-803-6025 or 1-954-677-4599. © 2010 Home Diagnostics, Inc. TRUEresult and TRUEtest are trademarks of Home Diagnostics, Inc. E9SUN21 Rev. 3 • E9051-90...
-
Page 3: Safety Notice
Safety Notice TRUEresult Quality Assurance / Quality Control Manual ® ATTENTION: Pharmacy Staff, Nursing Staff, Laboratory Staff, and Central Supply Staff – Reminder of Potential for Falsely Elevated Blood Glucose Results due to Drug Interferences This Safety Notice is for personnel involved in the issuance of point-of-care blood glucose testing systems and personnel involved in actual point-of-care blood glucose testing. -
Page 4: Safety Notice
If your facility provides a blood glucose monitoring system for use at home for an at-risk patient, instruct the patient not to use a blood glucose monitoring system that utilizes GDH-PQQ, including the TRUE2go and TRUEresult Systems, • After drug treatment is completed, ensure that use of a blood glucose monitoring system that utilizes GDH-PQQ is cleared by the Doctor or Healthcare Professional before use. -
Page 5: Introduction
DO NOT change patient medication, diet, or exercise routine without consulting the patient’s Doctor or Diabetes Healthcare Professional. Use of TRUEresult in a manner not specified in this Manual is not recommended and may affect the ability to determine true blood glucose levels. -
Page 6: Table Of Contents
Section 9: Training Certification Program Certificate Information ......................46-47 Training Checklist ........................48-49 Training Written Test ......................50-54 Training Certificate (example)......................55 Section 10: Forms Quality Control Log.........................57-58 At-Risk Patient Identification Notice ...................59 Section 11: TRUEresult Lifetime Warranty TRUEresult Lifetime Warranty ....................61 Section 12: References References .............................63... -
Page 7: Section 1: Using Trueresult In A Clinical Setting
Section 1: Using TRUEresult ® in a Clinical Setting... -
Page 8: Critical Safety Information/Important Information/Limitations/Expected Results
2 levels of Control. For testing frequency and the number of Control levels to test, refer to your facility quality control procedure/policy. TRUEresult is an in vitro quantitative system that is used for self • testing and point-of-care testing of human whole blood only. - Page 9 Results from the TRUEresult System are considered accurate if within + 20% of laboratory results. If patient has recently eaten, finger results from the TRUEresult System can be up to 70 mg/dL higher than venous laboratory results. •...
- Page 10 TRUEresult Quality Assurance / Quality Control Manual ® Expected Results Each patient should have specific blood glucose target ranges that are determined by the Doctor or Diabetes Healthcare Professional. Having most blood glucose results within the patient’s target range shows how well a treatment plan is working to control glucose levels.
-
Page 11: Regulatory Requirements - Clia
TRUEresult Quality Assurance / Quality Control Manual ® Regulatory Requirements - CLIA Self-testing and point-of-care testing of blood glucose has been classified by the Clinical Laboratory Improvement Amendments (CLIA) as a waived test. CLIA requires all entities that perform even one test, including waived tests, [on... -
Page 12: Proficiency Testing Information
The proficiency testing only serves to show how your results compare to other TRUEresult system users. If your proficiency sample results are not within acceptable limits of other TRUEresult users’... -
Page 13: Meter Specifications
TRUEresult Quality Assurance / Quality Control Manual ® Meter Specifications Result Range: 20 - 600 mg/dL Sample Size: Minimum 0.5 microliters (0.5 µL) Sample Type: Fresh capillary whole blood from the finger or forearm, venous blood drawn in EDTA (purple top tube) or heparin... -
Page 14: Material Safety Data Sheets (Msds)
TRUEresult Quality Assurance / Quality Control Manual ® Material Safety Data Sheet Section 1 : Product Information Product Name : TRUEtest Blood Glucose Test Strips Date Prepared : 16 April 2008 Revision Number : 0 Section 2 : Composition / Information on Ingredients... - Page 15 TRUEresult Quality Assurance / Quality Control Manual ® Section 6 : Spill or Leak Procedures Steps to be taken in case material is released or spilled: Contain material to prevent contamination of soil, surface water and ground water. May be slipping hazard.
- Page 16 TRUEresult Quality Assurance / Quality Control Manual ® Section 11 : Toxicological Information Chronic Effects of Overexposure: None currently known. Carcinogen or Suspected Carcinogen: None of the compounds present are listed as a carcinogen or suspected carcinogen. Medical Conditions Aggravated by Exposure: None currently known.
- Page 17 TRUEresult Quality Assurance / Quality Control Manual ® Material Safety Data Sheet Section 1 : Product Information Product Name : TRUEtest Glucose Control Solution - Levels 1, 2, and 3 Date Prepared : 16 April 2008 Revision Number : 0...
- Page 18 TRUEresult Quality Assurance / Quality Control Manual ® Section 7 : Handling and Storage Store at temperatures and conditions as indicated on the product label. Keep bottle tightly closed when not in use. Section 8 : Personal Protection Ventilation: Use general room ventilation.
- Page 19 TRUEresult Quality Assurance / Quality Control Manual ® Section 12 : Ecological Information Ecological effects of this product have not been determined. Section 13 : Disposal Primary Container Type: Bottle with 3 mL Glucose Control Solution. Waste Disposal Method: Each disposal facility must determine proper disposal...
-
Page 20: Section 2: Description Of System
Section 2: Description of System... -
Page 21: Meter
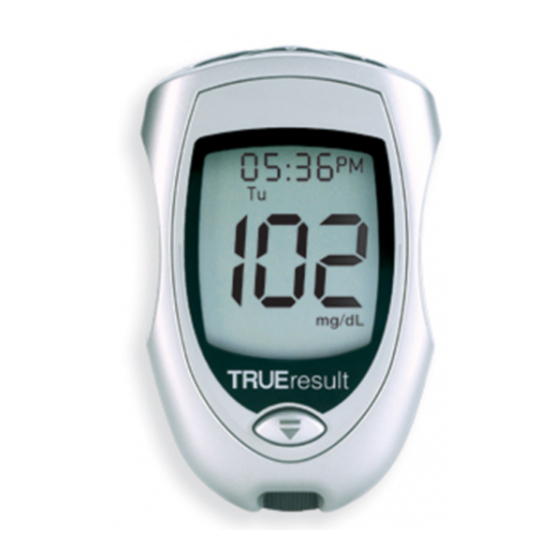
TRUEresult Quality Assurance / Quality Control Manual ® Description of System Meter Front Back Top: 1. “+” Button – Adds Alternate Site Symbol, increases numbers in Set Up, moves forward by time / date when viewing results in Memory. Note: Use of Memory function is not recommended in a multi-patient setting. -
Page 22: Truetest™ Test Strips
TRUEresult Quality Assurance / Quality Control Manual ® Full Display Full Display Components: 1. Result is from Memory. 2. Time, date 3. Time is AM/PM 4. Result is from 7, 14, or 30 day Average 5. Day of the week 6. - Page 23 TRUEresult Quality Assurance / Quality Control Manual ® Sample Placement Correct Placement Incorrect Placement • Allow sample (blood or Control) to be drawn into Sample Tip. • Do not smear or scrape sample with Tip of Test Strip. • Do not apply more sample to the Test Strip after removing the Test Strip from the sample drop.
-
Page 24: Truetest Glucose Control Solution
TRUEresult Quality Assurance / Quality Control Manual ® Glucose Control Solution Bottle Label 1. Lot Number (LOT) - Used for identification of lot for QC Form, used as a reference if calling for assistance. 2. Expiration Dates (EXP) - The printed Expiration Date is located on the Control bottle label next to EXP. -
Page 25: Section 3: Quality Control Testing
Section 3: Quality Control Testing... -
Page 26: Automatic Self-Test
TRUEresult is a no-coding system, which means the Meter does not have to be coded to each lot of Test Strips. To assure accurate and reliable results, TRUEresult offers two kinds of Quality Control Tests. -
Page 27: How To Perform A Control Test
TRUEresult Quality Assurance / Quality Control Manual ® How to Perform a Control Test Use the Quality Control Log located in Forms, Section 10 to record Control Test results. 1. Allow Control bottle, vial of Test Strips and Meter to adjust to room temperature (59°... - Page 28 System should not be used for testing blood. Call for assistance (see cover for phone number). 11. Record the result in the TRUEresult Quality Control Log (see Forms, Section 10). 12. Hold the Test Strip vial with Test Strip pointing down. Press the Strip Release Button to release and discard the Test Strip into an appropriate container.
-
Page 29: Section 4: Blood Glucose Testing
Section 4: Blood Glucose Testing... -
Page 30: Sample Information
TRUEresult System. Laboratory tests should be performed within 30 minutes of a blood glucose meter test to minimize the changes in glucose values due to glycolysis. -
Page 31: Obtaining A Blood Sample
® ® ® Using the TRUEresult System for testing patients with the above situations may result in falsely high glucose results. A falsely elevated glucose result may cause a patient or healthcare professional to take appropriate steps to bring the blood glucose in normal range, including giving insulin. The inappropriate use of insulin could lead to unconsciousness, severe hypoglycemic coma and possible death. -
Page 32: How To Perform A Blood Test
Close Test Strip vial immediately by pressing firmly down on the top of the vial cap. Note: The TRUEresult is a no-coding system, which means the Meter does not have to be coded to each lot of test strips. -
Page 33: System Out Of Range Warning Messages
The Ketone Alert may be turned on or off during Set Up, Section 5. System Out of Range Warning Messages WARNING! The TRUEresult System accurately reads blood glucose levels from 20 - 600 mg/dL. If test result is less than 20 mg/dL, “Lo” appears in the Meter Display. -
Page 34: Section 5: Set Up Of Time, Date, Ketone Alert And Testing Reminders
Section 5: Set Up of Time, Date, Ketone Alert and Testing Reminders... -
Page 35: Time/Date
TRUEresult Quality Assurance / Quality Control Manual ® Set Up of Time, Date, Ketone Alert, Testing Reminders Note: Setting up the time, date, Ketone Alert and Testing Reminders may not be suitable for a multi-patient use of the System. Check with the facility procedures and policies before performing Set Up. -
Page 36: Ketone Alert
TRUEresult Quality Assurance / Quality Control Manual ® Ketone Alert Note: If the Meter turns off at any time during the Set Up, go back to Step #1 under Time/Date and begin again. When a blood glucose result is over 240 mg/dL, the Ketone Alert is a reminder to check the patient’s ketones per their treatment plan prescribed by Doctor or... -
Page 37: Exit Set Up
TRUEresult Quality Assurance / Quality Control Manual ® 4. After the time is set by pressing the “S” Button, the Meter goes to the next Testing Reminder set-up (A-2). Repeat turning on and setting the time (see Steps 1 - 3) for the next 3 Reminders (if needed). -
Page 38: Section 6: Memory
Section 6: Memory... -
Page 39: Memory, Viewing Averages, Viewing Memory
TRUEresult Quality Assurance / Quality Control Manual ® Memory Note: The use of the Memory features (Averages, Memory) may not be suitable for a multi-patient use of the System. Check with the facility procedures / policies before use. Viewing Averages The Averages feature allows the viewing of the average of all the blood glucose results performed on the Meter within a 7, 14 or 30 day period. - Page 40 System. Check with the facility procedures / policies before use. The TRUEresult Meter’s Memory stores 500 results. The oldest glucose result is removed from the Memory when the Memory is full and a new glucose result is added.
-
Page 41: Section 7: Care And Storage Of System
Section 7: Care and Storage of System... -
Page 42: System (Meter And Test Strips)
TRUEresult Quality Assurance / Quality Control Manual ® Caring for the TRUEresult System • Store the System (Meter, Test Strips, Glucose Control Solution) in an area protected from liquids, dust and dirt. • Store the System in a dry place at room temperature, 36° - 86°F. -
Page 43: Battery Replacement
TRUEresult Quality Assurance / Quality Control Manual ® Battery Replacement The Low Battery Symbol displays if the battery needs to be changed. The Meter will continue to function for about 50 more tests before Meter will not test. A dead battery displays the Battery Symbol, beeps, and then turns off. Use only a new 3V non-rechargeable lithium battery (#CR2032) 1. -
Page 44: Section 8: Troubleshooting
Section 8: Troubleshooting... -
Page 45: Display Messages
Troubleshooting The following is a brief guide for Troubleshooting the most common errors when using TRUEresult. If any problems arise that cannot be resolved by using the guide or the Display Messages, please call for assistance. 1) After inserting Test Strip into Test Port, Meter does not turn on. -
Page 46: Display Messages
TRUEresult Quality Assurance / Quality Control Manual ® Display Messages Display Reason Action Temperature Error Move System to area between 50°-104°F and wait 30 Too cold or too hot for minutes for System to reach room temperature before testing. testing. -
Page 47: Section 9: Training Certification Program
Section 9: Training Certification Program... -
Page 48: Certificate Information
Glucose Monitoring System for point-of-care multi-patient facilities. The Training Certificate provides a record that the person listed on the Certificate has been trained correctly in the use of the TRUEresult System and understands all procedures and limitations concerning the TRUEresult System. - Page 49 1. Watch the TRUEresult Clinical Training Video (if available). 2. Successfully complete the Training Checklist under the instruction of a Certified Trainer. 3. Demonstrate the proper technique for testing with the TRUEresult System using the appropriate blood and Control samples.
-
Page 50: Training Checklist
____ CLIA regulations for point-of-care blood glucose testing ____ System specifications ____ Limitations and critical safety information, including that the TRUEresult System must not be used for certain patients (neonates, peritoneal dialysis patients, etc.) 3. Familiarization with the components of the System. -
Page 51: Training Checklist
Control Test result is not within the acceptable range ____ Demonstrates the procedure using the Glucose Control Solution ____ Records the Control Test result on the TRUEresult Quality Control Log 5. Blood Collection ____ Understands the proper technique of capillary blood collection for both finger and forearm samples... -
Page 52: Training Written Test
Address _________________________________________________________________________ Phone/Fax _______________________________________________________________________ E-mail Address ___________________________________________________________________ True or False 1. All Healthcare Professionals performing blood glucose monitoring using the TRUEresult System should complete the training program. __________True __________False 2. The TRUEresult System can be used on newborns. - Page 53 8. The battery should be replaced with an AAA alkaline battery. __________True __________False Multiple Choice (choose only one answer for each question) 1. Training on the use of the TRUEresult System consists of reviewing the following: a) The Clinical Training Video b) The QA/QC Manual c) The Owner’s Booklet...
- Page 54 TRUEresult Blood Glucose System Certified Trainer Post Test (Please print) ® Name_______________________________________________________ 4. The TRUEresult System must not be used for the following patient conditions: a) Peritoneal dialysis patients receiving dialysis solutions containing icodextrin (e.g. Extraneal , Icodial ) that is metabolized to maltose ®...
- Page 55 Blood Glucose System Certified Trainer Post Test (Please print) ® Name_______________________________________________________ 8. The following tests are used for Quality Control of the TRUEresult: a) One level of Glucose Control and a patient sample b) One level of Glucose Control c) A low level of Glucose Control and a high level of Glucose Control d) Meter automatic self-test and a minimum of 2 levels of Glucose Control 9.
- Page 56 TRUEresult Quality Assurance / Quality Control Manual ® 14. If after inserting the Test Strip into the Test Port, the Meter does not turn on, the reason could be a) The Test Strip was inserted upside down b) The Test Strip was not fully inserted...
-
Page 57: Training Certificate (Example)
TRUEresult Quality Assurance / Quality Control Manual ®... -
Page 58: Section 10: Forms
Section 10: Forms... -
Page 61: At-Risk Patient Identification Notice
Quality Assurance / Quality Control Manual ® At-Risk Patient Identification Notice (For patient bedside, with patient chart or in applicable treatment areas) Do not use TRUEresult System for Blood Glucose Testing! _______ Patient on peritoneal dialysis _______ Patient receiving solutions containing galactose or... - Page 62 Section 11: TRUEresult ® Lifetime Warranty...
-
Page 63: Trueresult Lifetime Warranty
TRUEresult Limited Lifetime Warranty Home Diagnostics, Inc. provides the following Warranty to the original purchaser of the TRUEresult Blood Glucose Meter: 1) Home Diagnostics, Inc warrants this Meter to be free of defects in materials and workmanship at the time of purchase. If the Meter is ever inoperative, Home Diagnostics, Inc. -
Page 64: Section 12: References
Section 12: References... -
Page 65: References
TRUEresult Quality Assurance / Quality Control Manual ® References 1. U.S. Food and Drug Administration, Center for Biologics Evaluation and Research. Important Safety Information on Interference With Blood Glucose Measurement Following Use of Parenteral Maltose/Parenteral Galactose/Oral Xylose-Containing Products. [Electronic Version]. Retrieved June 30, 2009.


Need help?
Do you have a question about the TRUEresult and is the answer not in the manual?
Questions and answers