Fresenius Kabi Amika Instructions For Use Manual
Enteral feeding pump
Hide thumbs
Also See for Amika:
- Instructions for use manual (69 pages) ,
- Pictorial reference manual (2 pages) ,
- Reference manual (2 pages)
Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Fresenius Kabi Amika
- Page 1 Amika Enteral feeding pump Version 2.3 / i Instructions For Use...
- Page 2 Symbol descriptions Medical Device Unique Device Identifier Refer to the Instructions For Use CE marking Class II equipment Mass; weight Product reference Product serial number Name and address of the manufacturing Manufacturer facility Battery characteristics Defibrillation-proof type CF applied part Direct Current (DC) Alternating Current (AC) Electrical output...
- Page 3 INFORMATION Please refer to the Use environment section for additional information on temperature, pressure and humidity limitations.
-
Page 4: Table Of Contents
Table of contents 1 Introduction 1.1 Scope........................7 1.2 Principles of operations..................7 1.3 Intended purpose....................7 1.4 Intended use......................7 1.4.1 Indications....................7 1.4.2 Contraindications..................7 1.4.3 Intended users....................8 1.4.4 Intended patients..................8 1.4.5 Use environment..................8 1.5 Clinical benefits.....................9 1.6 Side-effects......................9 1.7 Risks for patients....................9 2 Description 2.1 System definition....................10 2.2 Packaging content....................10 2.3 General description.....................10... - Page 5 4.2.1 Switch-on....................20 4.2.2 Installing the giving set................20 4.2.3 Priming the giving set................23 4.2.4 Change feeding setting................25 4.2.5 Start feeding.....................26 4.2.6 Terminate feeding..................26 4.2.7 Switch off pump..................27 4.2.8 Removing/Changing the giving set from the pump........28 4.2.9 Keypad lock....................29 4.2.10 Mute alarm....................29 5 Pump menu 5.1 Access menus.....................31 5.2 Feeding mode......................32...
- Page 6 8.1.2 Flow Rate range..................50 8.1.3 Volume range...................50 8.1.4 Upstream and downstream occlusions.............50 8.1.5 Volume Accuracy..................51 8.1.6 Empty bag / Air in Line alarm response time at different flow rates..51 8.1.7 Giving set alarm response time at different flow rates......51 8.2 Technical characteristics..................51 8.2.1 Operation mode..................51 8.2.2 Power supply specifications..............52 8.2.3 Battery specifications................52...
-
Page 7: Introduction
Amika is an enteral feeding pump with a holder power and disposables dedicated to enteral feeding and hydration. The intended use of Amika pump and sets is to deliver nutrition and hydration fluids to the patient through a feeding tube, in a safe, instinctive and convenient manner. -
Page 8: Intended Users
Backpack, in pre-hospital medical ground transportation and in homecare. INFORMATION For Thailand, homecare is not the claimed use environment for Amika pump. The Amika power cable is not meant to be used outdoors (such as in the garden, on the patio). WARNING ■... -
Page 9: Clinical Benefits
1.5 Clinical benefits The therapeutic benefit of Amika enteral feeding pump for the patient is enabling controlled and safe enteral feeding in a clinical and outpatient setting as well as mobile. The objective of enteral nutrition is prevention and treatment of malnutrition to improve outcome. -
Page 10: Description
2 Description 2.1 System definition The Amika system is composed of the following components: ■ Amika pump: enteral feeding pump with pump holder and power cable. ■ Amika disposable (applied part): giving sets. ■ Amika accessories. For more information on accessories, refer to their respective accompanying documents. -
Page 11: Detailed Description
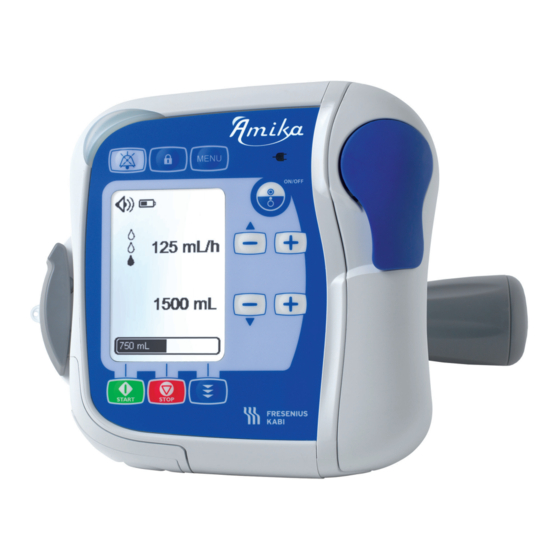
2.4 Detailed description Pump description Legend Tube guides Pinch clamp slot Pumping mechanism Status light indicator Front panel (keypad) Door lever Pump door Pump identification label Speaker Rails for installation on pump holder Contact pins for pump to holder connection Pump door identification label... - Page 12 Pump holder description Legend Clamp handle Contact pins for pump to holder connection Slot Grey locking lever Mains supply light indicator Pole clamp Holder identification labels Power cable inlet Mains supply light indicator, on the front panel of the holder Near the power cable inlet of the holder, description in Power supply...
-
Page 13: Display Description
2.5 Display description Status Bar icons Sound level icons Alarm icon Battery icon Muted alarm icon Keypad locked icon Settings lock icon Setting screen layout Legend Pumping status indicator: Pumping is stopped Pumping is in progress Status bar Flow rate Target volume Progress bar showing volume delivered... -
Page 14: Installation And Removal
3 Installation and removal Installation and removal must only be done when the patient is not connected. Check that the Amika pump, holder and power cable are not damaged in any way at the beginning of installation and at the end of removal. -
Page 15: Using The Pole Clamp
3.1.2 Using the pole clamp The holder can be attached universally, vertically and horizontally. Turn the pole clamp to the suitable position. 3.1.3 Positioning the holder on a rail, pole, bed or wheelchair Ensure the holder is positioned so that the display is at the suitable height to ensure good visibility and orientation in the reading direction (the contact pins are at the bottom). -
Page 16: Positioning The Pump
3.1.5 Positioning the pump Slide the pump down until the grey locking lever locks the position. 3.1.6 Electrical connection Ensure power cable is not damaged. To charge battery or to use the pump on the mains power supply: 1. Connect power cable to the holder. 2. -
Page 17: Removal
3.2 Removal 3.2.1 Removing the pump from the pump holder 1. Push the grey locking lever. 2. Pull the pump up. 3.2.2 Removing the pump holder 3.2.3 Electrical disconnection 1. Remove power cable from power socket. ■ A beep is emitted by the pump with mains supply light indicator turned off when the power cable is disconnected. -
Page 18: Attaching / Removing The Quick Guide
2. Remove power cable from holder. 3.2.4 Attaching / Removing the Quick Guide A quick guide can be easily attached and removed from the pump holder. -
Page 19: Operations
( flashing). ■ If battery is failing, do not use the device. Return device to Fresenius Kabi sales representative as soon as possible. ■ Battery replacement must be performed by qualified and trained technical personnel in compliance with the technical manual and procedures. -
Page 20: Basic Operations
In order to protect user health, please follow clean aseptic handling procedures for container, set or feeding tube disposal. WARNING ■ Only Fresenius Kabi giving sets can guarantee pump reliability. Please refer to the compatible giving sets (see Giving sets... - Page 21 ■ Check the giving set's intended use regarding the feeding protocol, especially for patients requiring special attention. ■ Check giving set and patient connection integrity before use. CAUTION The fluid in the giving set and the bag/bottle must be within normal temperature conditions: +10°C to +40°C.
- Page 22 2. Position the pinch clamp using the arrow marks indicating the direction of the flow Insert the pinch clamp until you hear the ‘CLIC’ 3. On the sides of the pump, place the tube straight inside tube guides. WARNING Check that the giving set is correctly installed to avoid patient harm such as overfeeding, underfeeding.
-
Page 23: Priming The Giving Set
■ automatic priming: Amika pump automatically fills in the giving set at maximum rate by depressing the automatic priming key ■ semi-automatic priming: Amika pump fills in the giving set at maximum rate as long as the semi-automatic priming key is kept depressed. - Page 24 Automatic priming Auto priming can be stopped at any time: At the end of automatic priming, it is possible to continue the priming using the semi-automatic priming function as defined below. Semi-automatic priming Press key to access to the priming modes. Press key to launch the priming.
-
Page 25: Change Feeding Setting
WARNING At the end of priming, check that the set is correctly primed. 4.2.3.2 Priming without the pump (Manual priming) Remove the giving set from the pump (see Removing/Changing the giving set from the pump on page 28). 1. Close pinch clamp. 2. -
Page 26: Start Feeding
■ Adjust target volume (mL) Press key to set the target volume. WARNING Make sure feeding parameters are checked before starting feeding (programming error can lead to overfeeding, underfeeding or delay of therapy). 4.2.5 Start feeding 1. Connect the giving set to the patient's enteral feeding tube. 2. -
Page 27: Switch Off Pump
When feeding is stopped, flow rate and target volume parameters can be adjusted. Then, feeding can resume. ■ Reset the progress bar. When the pump is stopped, progress bar can be reset by depressing the key for 2 seconds. 4.2.7 Switch off pump Feeding shall be stopped before switching off the pump. -
Page 28: Removing/Changing The Giving Set From The Pump
INFORMATION ■ When feeding is on-going, the key is inactive: the forbidden key beep is triggered but feeding continues. ■ When switched off, the pump retains the following information: – flow rate, volume and progress bar on the setting screen; –... -
Page 29: Keypad Lock
Install a new giving set in the pump (see Installing the giving set on page 20). 4.2.9 Keypad lock Keypad lock prevents from unintentional tampering of pump settings. Keypad can be locked / unlocked by depressing the keypad lock key for 2 seconds. - Page 30 When a low priority alarm is muted: ■ the mute icon is displayed in the status bar; ■ the alarm symbol is displayed and the yellow LED is lit; ■ the alarm sound is off and an information signal sound (2 beeps) is emitted every 30 minutes.
-
Page 31: Pump Menu
5 Pump menu INFORMATION ■ The menu is accessible when feeding is stopped. ■ A beep sound is triggered when a forbidden key (not active in specific screens) is depressed. ■ During a procedure, press ) to validate the choice and go back to the setting screen. -
Page 32: Feeding Mode
Menu navigation Press then press to scroll up / down between submenus. Press to enter the submenu. 5.2 Feeding mode On this screen, target volume is activated . If you programme a feeding with no target volume and a feeding with target volume with respectively different flow rates, the respective flow rates are saved. -
Page 33: Night Mode
5.3 Night mode On this screen, night mode is activated Press to select Night or Day Mode. Press to activate Day Mode or activate Night Mode. Press to validate Night or Day Mode. INFORMATION ■ When night mode is activated, the brightness of mains supply light and screen will be decreased. -
Page 34: Settings Lock
When settings lock is activated: ■ is displayed in the status bar; ■ target volume and flow rate cannot be changed; ■ Accessible keys are: with restrictions. INFORMATION ■ To get the access code, contact your Fresenius Kabi sales representative. -
Page 35: Cumulative Feeding Volume Counter
■ Settings lock activation / deactivation isn’t modified after switching OFF the pump. ■ When settings lock is activated, keypad lock can still be activated / deactivated. 5.6 Cumulative feeding volume counter Press to display the cumulative feeding volume. The total feeding volume since last reset is displayed. -
Page 36: Feeding History
Press to display the alarm events. Press to switch from one alarm event to another. INFORMATION ■ Alarm history indicates the type of alarm and the time elapsed since the event happened. Example: a battery empty alarm occurred 9 days, 15 hours and 22 minutes ago. -
Page 37: Contrast / Brightness
INFORMATION ■ Feeding history indicates the delivered volumes, their associated flow rate and the time elapsed since their delivery. Example: a volume of 1500 mL was administered at a flow rate of 125 mL/h, 13 days, 2 hours and 25 minutes ago. ■... -
Page 38: Set Time For Target Volume Almost Reached Message
■ The access code is required to set time between two alarm sounds. WARNING Time between 2 alarms can be adjusted from 2.5 to 30 seconds with steps of 0.5 seconds. This adjustment can modify the perception of an alarm (Default value 2.5 seconds). -
Page 39: Reset Manufacturing Settings
Press to access the technical information menu. NOTE: the technical information menu displays: Pump serial number Software version/ Hardware version Production date (mm/dd/yyyy) Last maintenance date (mm/dd/yyyy) Total delivered volume Total functioning time 5.13 Reset manufacturing settings Reset manufacturing settings is recommended to facilitate the transition from one patient to another. -
Page 40: Cleaning And Disinfection
■ The pump is not intended to be sterilized, it may damage the pump. The Amika is a non-sterile medical device. ■ The Amika backpack must be cleaned before inserting the pump. Please refer to its specific accompanying documents. ■ Make sure to use the original door when replacing it on the pump (check the serial number on the pump is the same as on the door). -
Page 41: Cleaning Instructions
6.4.1 Cleaning Instructions Prerequisites ■ The pump is switched off. ■ The power cable and all other cables are unplugged. ■ The pump is disconnected from the holder. ■ The air is at room temperature (20 to 25 °C). ■ The operator is wearing suitable protective equipment. Protocol 1. -
Page 42: Disinfection Instructions
9. Make sure to use the original door when replacing it on the pump (check the serial number on the pump is the same as on the door). 6.4.2 Disinfection instructions Prerequisites ■ The cleaning protocol has been performed. ■ The pump is switched off. ■... -
Page 43: Alarms And Safety Features
7.1 Alarms / Actions The Amika pump has a continuous inspection system that operates as soon as it is in use. It is recommended that the user should be in front of the Amika pump, for best visibility of alarm display. -
Page 44: Alarm Descriptions
■ Check that the proper set is used (Amika giving sets only). ▷ See Installing the giving set on page Area where pinch clamp is inserted is ■... - Page 45 Symbol Meanings Actions ■ Close pump door. Door open Pump door not properly closed at start. ▷ See Installing the giving set on page Pump door opened after start. ■ Close pump door. ▷ See Installing the giving set on page Pump door removed from its anchoring.
- Page 46 Symbol Meanings Actions Target volume Target volume will be reached. The time of message before target almost reached volume is reached can be set in the menu. ▷ See Set time for target volume almost reached message on page 38. ■...
- Page 47 Symbol Meanings Actions Empty bag / Air in Feed container is empty. ■ End feeding or connect to a filled line feed container. Air is in the giving set. ■ Fill giving set to the end. ▷ See Priming the giving set on page Dirt in sensor area (lower tube guide).
-
Page 48: Maximum Alarm Raising Delay
Remove power cable smoking or with an ■ Do not use the device abnormally hot part. ■ Contact your biomedical department or Fresenius Kabi sales Pump screen, Holder power representative immediately or Holder COM are damaged Pump has been dropped ■... - Page 49 ■ Check fluid viscosity ■ Check the fluid is within normal temperature conditions ■ Contact your biomedical department or Fresenius Kabi sales representative if problem remains Front panel problem (keys, ■ Check the general state of the front panel (keypad) LEDs) ■...
-
Page 50: Technical Information
8 Technical information 8.1 Performance 8.1.1 Essential performance Essential pump performance is defined as follows in standard operating conditions: ■ flow rate accuracy (± 5% at 125 mL/h*); ■ occlusion detection time (< 6 min at 50 mL/h with medical water); ■... -
Page 51: Volume Accuracy
100 mL/h 15 s maximum 8.2 Technical characteristics 8.2.1 Operation mode The Amika pump is a reusable device. The pump ensures fluid delivery in a continuous feeding mode, using pumping and clamping fingers to push the liquid to the patient. -
Page 52: Power Supply Specifications
The test protocol used to obtain these results is described in 60601-2-24. The curves can be helpful in determining the suitability of feeding parameters for specific nutrition programmes. Giving set used: Amika Varioline Fluid used: distilled water, and Fresubin energy drink (1 mL/h only) - Page 53 8.2.6.1 Minimum flow rate: 1 mL/h Sampling time: 30 seconds Start up and instantaneous flow rate (1 mL/h, over first 2h of the test period) Trumpet curves for 2, 5, 11, 19, 31 minute observation windows (1 ml/h over second hour of the test period) Sampling time: 30 seconds...
- Page 54 Instantaneous rate (1 mL/h, over last 2 hours of set change interval, 24 hours) Trumpet curves for 2, 5, 11, 19, 31 minute observation windows (1 mL/h, over last hour of the set change interval, 24 hours) Sampling time: 15 minutes...
- Page 55 Instantaneous flow rate (1 mL/h, over set change interval 24 hours) Trumpet curves for 15, 60, 150, 330, 570, 930 minute observation windows (1 mL/h, over set change interval, 24 hours) 8.2.6.2 Intermediate flow rate: 25 mL/h Sampling time: 30 seconds Start up and instantaneous at intermediate flow rate (25 mL/h, over first 2h of the test period)
- Page 56 Trumpet curves for 2, 5, 11, 19, 31 minute observation windows (25 mL/h over second hour of the test period) Instantaneous rate (25 mL/h, over last 2 hours of set change interval, 24 hours)
-
Page 57: Compliance With Standards
Trumpet curves for 2, 5, 11, 19, 31 minute observation windows (25 mL/h, over last hour of the set change interval, 24 hours) Sampling time: 15 minutes Instantaneous flow rate (25 mL/h, over set change interval 24 hours) Trumpet curves for 15, 60, 150, 330, 570, 930 minute observation windows (25 mL/h, over set change interval, 24 hours) 8.2.7 Compliance with standards General requirements for basic safety and essential... - Page 58 General requirements, tests and guidance for alarm Conform to IEC 60601-1-8 systems in medical electrical equipment and medical electrical systems Requirements for medical electrical equipment and Conform to IEC 60601-1-11 medical electrical systems used in the home healthcare environment Conform to the Medical Device Regulation (EU) 2017/745 0123 : Notified body number (TÜV SÜD Product Service GmbH, Ridlerstrasse.
-
Page 59: Transport, Storage And Recycling Conditions
9 Transport, storage and recycling conditions 9.1 Storage and transport conditions During transport, the Amika pump shall not be removed from its pole or rail when carrying feeding devices, especially when feeding is running. Check that the power cable is connected and operational after transport of the pump. -
Page 60: Install The Device After Storage
They must be collected separately and disposed of according to local regulations. Before disposal, make sure that a qualified technician removes the battery from the device according to the procedure described in the Technical Manual. For further information on waste processing regulations and dismantling, please contact your Fresenius Kabi sales representative. -
Page 61: Guidance And Manufacturer's Declaration On Emc
10 Guidance and manufacturer’s declaration on The Amika pump is intended to be used in the electromagnetic environment specified below. The customer or the user of the Amika pump should ensure that it is used in such an environment. Excluding the cases described in this manual, the pump operation must systematically be checked by a qualified operator, should the pump be installed in the vicinity of other electrical devices. -
Page 62: Guidance And Manufacturer's Declaration - Electromagnetic Immunity
UPS circuit (uninterruptible power supply); ■ increase the separation between the Amika and patient or disruptive equipment; ■ connect the Amika into an outlet on a different circuit from that to which the patient or disruptive equipment is connected; ■ in any case, whatever the context, the user should conduct interoperability testing in a real situation to find the right setup and good location. -
Page 63: Services
Please contact your Fresenius Kabi sales representative for additional information. 11.2 Quality control Upon request by the hospital, a quality control check can be performed on the Amika every 12 months. A regular quality control (not included in the guarantee) consists of various inspection operations (including the functionality check of alarm system) listed in the technical manual. -
Page 64: Maintenance Requirements
■ When replacing components, only use Fresenius Kabi spare parts. ■ When using the device on a patient, no maintenance action must be performed. Life cycle of Amika pump: 10 years provided that the maintenance is properly performed as described above. 11.4 Service policy and rules For further information concerning device servicing or use, please contact our sales representative or our Customer service. - Page 65 GERMANY Tel.:+49 (0) 6172 / 686-0 http://www.fresenius-kabi.com...
-
Page 66: Ordering Information
Fresenius Kabi sales representative for orders. 12.2 Giving sets Do not use Amika giving sets to deliver liquids using gravity method, except the Amika set Varioline Comfort that can be used either for feeding by pump or by gravity. - Page 67 Accessories Reference Smart Holder Power EU Accessory CS1000428 Smart Holder COM EU Accessory CS1000429 Please contact your Fresenius Kabi sales representative for orders.
-
Page 68: Glossary Of Terms
13 Glossary of terms Term Description °C Celsius Degree Alternating Current Ampere hours Amika Enteral feeding and hydratation pump manufactured by Fresenius Kabi CE marking European Conformity Marking CISPR International Special Committee on Radio Interference Centimeters Decibel DECT Digital Enhanced Cordless Telecommunications... - Page 69 Term Description mL/h Milliliter per hour Millimeters Magnetic Resonance Imaging NiMH Nickel-Metal Hydride Nuclear Magnetic Resonance Random Access Memory Radio Frequency RFID Radio Frequency Identification Read-Only Memory Seconds Uninterruptible Power Supply Volt Volt Alternating Current Volt Direct Current Watt...
- Page 70 ■ Addition of a new function "Reset manufacturing settings"; ■ Replace the related information and drawings of "Amika holder" in the whole IFU with "Smart holder power"; ■ Change the accuracy from "±7% at 50 mL/h" to "±5% at 125 mL/h" in chapter 9.1.1 and 9.1.2;...
- Page 71 This document may not be reproduced in whole or in part without the written consent of Fresenius Kabi. Amika® is a registered trademark in the name of Fresenius Kabi in selected countries.
- Page 72 Local contacts for servicing Fresenius Kabi AG Fresenius Kabi (Nanchang) Else-Kröner-Str. 1 CO., Ltd. 61352 Bad Homburg Qin Lan Road, Nanchang GERMANY Economic & Technological Tel.: +49 (0) 6172 / 686-0 Development Zone, 330013 http://www.fresenius-kabi.com Nanchang, Jiangxi Province PEOPLE'S REPUBLIC OF...









Need help?
Do you have a question about the Amika and is the answer not in the manual?
Questions and answers