Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for W&H implantmed PLUS SI-1010
- Page 1 Instructions for Use Edition USA SI-1010 / SI-1015 / SI-1023...
-
Page 2: Table Of Contents
Contents Symbols .......................................... 4 1. Introduction ....................................... 8 2. Unpacking ........................................ 10 3. Scope of delivery ..................................... 11 4. Safety notes ......................................12 5. Description........................................17 of front panel ......................................17 of rear panel ......................................18 of foot control S-N2/S-NW .................................. 19 6. Start-up ........................................21 7. - Page 3 Contents 10. Hygiene and maintenance ................................... 41 General notes ...................................... 41 Limitations on processing .................................. 42 Initial treatment at the point of use ..............................43 Manual cleaning ....................................44 Manual disinfection .................................... 45 Automated cleaning and disinfection ............................... 46 Drying ........................................47 Inspection, maintenance and testing ...............................
-
Page 4: Symbols
Symbols WARNING! Sterilizable up to the stated Consult Instructions for Use (if persons could be injured) temperature ATTENTION! CE marking with identification Do not dispose of with (if property could be damaged) number of the Notified Body domestic waste XXXX Manufacturer General explanations, without DataMatrix code for product... - Page 5 Symbols Follow Instructions for Use Electric fuse Frequency of the alternating current Class II equipment Earth MEDICAL – GENERAL MEDICAL EQUIPMENT AS TO ELECTRICAL 25UX SHOCK, FIRE AND MECHANICAL HAZARDS ONLY IN ACCORDANCE WITH ANSI/AAMI ES60601- Foot control Supply voltage 1:2005/(R)2012 + A1:2012 + of the control unit C1:2009/(R)2012 + A2:2010/...
- Page 6 Symbols Revolutions per minute Temperature limitation Caution! According to +70 °C (+158°F) Max. (= rpm) Federal law restricts this device to sale by or on the -40 °C (-40°F) Min. order of a physician, dentist, veterinarian or with the This way up Humidity limitation descriptive designation of any 80 %...
- Page 7 Symbols Batch code Use by Latex-free Not for re-use Do not use when package is Sterilization with ethylene damaged oxide Do not resterilize Keep away from heat Single sterile barrier system...
-
Page 8: Introduction
1. Introduction For your safety and the safety of your patients These Instructions for use explain how to use your medical device. However, we must also warn against possible hazardous situations. Your safety, the safety of your team and, of course, the safety of your patients, are of paramount importance to us. Observe the safety notes. - Page 9 Introduction Responsibility of the manufacturer The manufacturer can only accept responsibility for the safety, reliability and performance of the medical device when compliance with the following instructions is ensured: > The medical device must be used in accordance with these Instructions for Use. >...
-
Page 10: Unpacking
2. Unpacking Remove the packaging. Remove the foot control, Instructions for Use and accessories. Remove the motor with cable. Lift out the insert with the control unit. Remove the mains cable, irrigant support, universal support, irrigation tubing set and Instructions for Use. -
Page 11: Scope Of Delivery
3. Scope of delivery SI-1023 (230V) SI-1015 (120V) SI-1010 (100V) Control unit 30288000 30289000 30290000 Irrigation tubing set 2.2 m REF 436360 (3 pcs, disposable) REF 07721800 Universal support REF 04005900 Irrigant support Mains cable country-specific Optional included in set EM-19 LC motor with electrical REF 3028100x contacts and 1.8 m or 3.5 m cable... -
Page 12: Safety Notes
4. Safety notes > Before using the medical device for the first time, store it at room temperature for 24 hours > Check the medical device for damage and loose parts each time before using. > Do not operate the medical device if it is damaged. >... - Page 13 Safety notes > Use only original W&H fuses. > Never touch the patient and the electrical contacts on the control unit simultaneously. > Make sure that no computer viruses are transferred to the control unit by an external data medium (USB stick). The connection of a USB hard drive with an external power source is not permitted.
- Page 14 Safety notes Mains cable / Power switch > Only use the mains cable supplied. > Plug the mains cable only into an earthed power socket. > Set up the control unit so the power switch and the socket are easily accessible at all times. Disconnect the control unit from the power supply in case of danger.
- Page 15 Safety notes Risks due to electromagnetic fields The functionality of active implantable medical devices (AIMD) (e.g. cardiac pacemaker, ICD) can be affected by electric, magnetic and electromagnetic fields. > Find out if the patient has active implantable medical devices (AIMD) before using the medical device and inform about the risks.
- Page 16 Safety notes Coolant supply The medical device is designed for use with physiological saline solution. > Always ensure correct operating conditions and that sufficient and adequate coolant is delivered. > Always provide sufficient coolant and ensure the appropriate suction. > Use only suitable coolants and follow the manufacturer’s medical data and instructions. >...
-
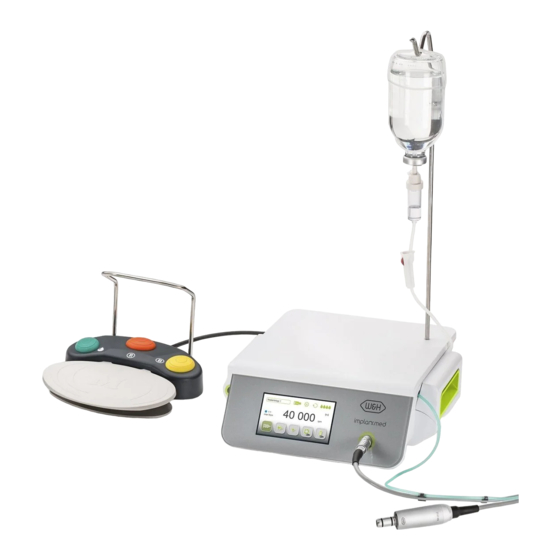
Page 17: Description
5. Description of front panel Irrigant support locator Pump cover Display (touchscreen) Pump cover OPEN Connection for motor... -
Page 18: Of Rear Panel
Description of rear panel Irrigant support locator Connection for foot control Power switch ON/OFF Fuse holder with 2 fuses Connection for (2 x 250 V – T1.6 AH) mains cable... -
Page 19: Of Foot Control S-N2/S-Nw
Description of foot control S-N2/S-NW Locator ORANGE attach/detach Change program Change application GREEN Pump YELLOW ON/OFF Change motor direction Forward operation/reverse operation GREY Start motor (pedal) VARIABLE or ON/OFF (Factory setting = variable) - Page 20 Description of foot control S-N2/S-NW ORANGE S-N2 / S-NW: Change program Press the ORANGE button to change programs in ascending order. The motor direction is automatically set to forward operation every time the program is changed. When changing from the last program to the first program a longer acoustic signal sounds (risk of injury). GREEN –...
-
Page 21: Start-Up
6. Start-up Place the medical device on a flat level surface. Ensure that the medical device can be disconnected from the power supply at any time. Connect the mains cable and Attach the universal foot control. support and lock it. S-N2 Pay attention to the positioning! - Page 22 Start-up Switching on the control unit Switching off the control unit Plug the mains cable into an Switch off the control unit at earthed power socket. the power switch. Switch on the control unit Pull the power plug out at the power switch.
-
Page 23: Starting Operation
7. Starting operation Setup wizard The touch screen must only be touched using fingers. Using hard objects on the touch screen may scratch or damage the surface. Setting up Implantmed Switch on your control unit and follow the directions of the setup wizard. The set-up wizard guides you through the various set-up stages up to the main menu: >... -
Page 24: Control Unit Operation
8. Control unit operation Main menu My favorites Set program Documentation / Wi-Fi Pairing Set speed / torque Setup Foot control Forward/reverse operation mode ... - Page 25 Control unit operation My favorites Select drill protocol group An activated drill protocol cannot be deleted Edit > Adjust factory setting of drill protocol groups. > Create drill protocol Copy Rename Activate Delete...
- Page 26 Control unit operation Set program max. 50 Ncm Transmission Speed At 40,000 rpm the accuracy of the set speed is ±10 %. Torque Adjustment range 5 – 80 Ncm with WI-75 and WS-75 only. Adjustment range 5 – 70 Ncm with SZ-75 only. The motor switches off automatically when the set torque is reached in forward and reverse operation modes.
-
Page 27: Menue Navigation
Control unit operation Menue Navigation... - Page 28 Control unit operation User Torque curve An activated user cannot be deleted Set screen lock Add user Activating / deactivating screen lock Screen lock Manage user User settings: Copy, Rename, Activate, Delete Interval Interval: Select time Foot control Activating / deactivating LED Pairing –...
- Page 29 Control unit operation System check Wi-Fi-dongle Test run Dental numbering system Device info Select dental numbering system: FDI / UNS FDI (Féderation Dentaire Internationale = Service International dental numbering system) UNS (Universal Numbering System = Licenes American dental numbering system) GPL: GNU General Public License LGPL:...
- Page 30 Control unit operation Foot control Beacon Software update Beacon Pairing Reset Wi-Fi pairing Reset Restore factory settings Restart Control unit restarts automatically Import user settings Export user settings...
- Page 31 Control unit operation Confirm/save Drill function Favorite selected Drill function black = information Drill function green = Information with selection option red = error message, work cannot be continued Thread-cutter function orange = error message, work can be continued Implantatinsertion red = replace batteries Implant stability quotient measurement Foot control S-NW...
- Page 32 Control unit operation Factory settings Thread-cutter function (chip breaker mode) When the pedal (grey) on the foot control is pressed, the thread cutter rotates inwards until the set torque is reached. The control unit automatically switches to reverse operation when the set torque is reached. Disengaging and then re-engaging the pedal will switch the control unit back to forward operation.
-
Page 33: Documentation With Usb Stick
Control unit operation Documentation with USB stick Drill protocols, torque curves and ISQ values can only be documented in the thread-tapping function, implant insertion or ISQ measurement. Documentation must be activated or deactivated for each program. A USB stick is required to save the documentation. >... - Page 34 Control unit operation Documentation with USB stick Further documentation > Add new position > Start new docu > Complete docu When the motor stops, a diagram appears, which is automatically saved to the USB stick. Edit documentation A text file (csv) and a PDF file are saved on the USB stick. The text file can be opened in Microsoft®...
- Page 35 Control unit operation ioDent® platform Follow the directions and safety notes in the Instructions for Use of the ioDent® platform. Check the data exchange between the ioDent® platform and the medical device. Check the transferred data for completeness and correctness. Establishing a connection to the ioDent®...
-
Page 36: Iodent® Platform
Control unit operation ioDent® platform Connecting the medical device to an IT network or changing an IT network can lead to previously unidentified risks to patients, operators or third parties. The operator of the IT network is responsible for identification, analyzing, evaluating and controlling these risks. -
Page 37: Beacon
Control unit operation Beacon Follow the directions and safety notes in the Instructions for Use for the Beacon. Establishing a connection to the Beacon Insert the Osstell dongle. Beacon pairing (standard) > Only possible in the ISQ program. > All Beacons connect to the medical device automatically. Beacon pairing using the serial number >... -
Page 38: Error Messages
9. Error messages The error message disappears when it is clocked or when the pedal (grey) on the foot control is released. Icon Description of error Solution WARNING FOOT CONTROL > Check plug contacts of foot control > Check the plug contacts of the dongle WARNING MOTOR >... - Page 39 Error messages Icon Description of error Solution WARNING TIME-OUT > Release the pedal (grey) on the foot control > Allow motor to cool for at least 20 minutes SYSTEM ERROR > Switch the control unit off and back on again If the error message appears again, contact an authorized W&H service partner immediately.
- Page 40 Error messages Icon Description of error Solution WARNUNG CONNECTION > Press the ioDent® Wi-Fi dongle symbol > Attempt to establish a connection with the ioDent® platform again. WARNING DATA RECEPTION > Restart the data transfer on the ioDent® platform. WARNING TIME SYNCHRONISATION >...
-
Page 41: Hygiene And Maintenance
10. Hygiene and maintenance General notes Follow your local and national laws, directives, standards and guidelines for cleaning, disinfection and sterilization. Wear protective clothing, safety glasses, face mask and gloves. Use only oil-free, filtered compressed air with a maximum operating pressure of 3 bar for manual drying. Cleaning agents and disinfectants >... -
Page 42: Limitations On Processing
Hygiene and maintenance Limitations on processing The product lifetime and the medical device’s ability to operate correctly are mainly determined by mechanical stress during use and chemical influences due to processing. Send worn or damaged medical devices and/or medical devices with material changes to an authorized W&H service partner. -
Page 43: Initial Treatment At The Point Of Use
Hygiene and maintenance Initial treatment at the point of use > Clean and disinfect the medical device immediately after every treatment. > Wipe the control unit, the universal support and the irrigant support with disinfectant. Disconnect the power supply from the control unit. Check for any damage on the outer surface of each component. ... -
Page 44: Manual Cleaning
Hygiene and maintenance Manual cleaning Universal support / Irrigant support Do not immerse the medical product in liquid disinfectant or in an ultrasonic bath. Universal support / Irrigant support > Clean the universal support and the irrigant support under running tap water (< 35°C / < 95°F). >... -
Page 45: Manual Disinfection
Hygiene and maintenance Manual disinfection Universal support / Irrigant support W&H recommends wipe-down disinfection. Evidence of the basic suitability of the universal support and the irrigant support for effective manual disinfection (intermediate level) was provided by an independent test laboratory using the disinfectant “CaviWipes™” (Metrex). Control unit W&H recommends wipe-down disinfection. -
Page 46: Automated Cleaning And Disinfection
Hygiene and maintenance Automated cleaning and disinfection Universal support / Irrigant support W&H recommends automated cleaning and disinfection using a washer-disinfector (WD). Read the notes, follow the instructions and heed the warnings provided by the manufacturers of washer-disinfectors, cleaning agents and/or disinfectants. The control unit is not approved for automated cleaning and disinfection. -
Page 47: Drying
Hygiene and maintenance Drying Universal support / Irrigant support > Ensure that the universal support and the irrigant support are completely dry internally and externally after cleaning and disinfection. > Remove liquid residues using compressed air. -
Page 48: Inspection, Maintenance And Testing
Hygiene and maintenance Inspection, maintenance and testing Inspection – Universal support / Irrigant support > Check the medical device after cleaning and disinfection for damage, visible residual soiling and surface changes. > Reprocess any medical devices and stand that are still soiled. >... -
Page 49: Packaging
Hygiene and maintenance Packaging Universal support Pack the medical device in FDA sterilization packages that meet the following requirements: > The sterilization package must meet the applicable standards in respect of quality and use and must be suitable for the sterilization method. >... -
Page 50: Sterilization
Hygiene and maintenance Sterilization Universal support W&H recommends sterilization according to ANSI/AAMI ST55, ANSI/AAMI ST79. > Read the notes, follow the instructions and heed the warnings provided by the manufacturers of steam sterilizers. > The program selected must be suitable for the universal support. Recommended sterilization procedures >... - Page 51 Hygiene and maintenance Sterilization Evidence of the medical device’s basic suitability for effective sterilization was provided by an independent test laboratory using the LISA 517 B17L* steam sterilizer (W&H Sterilization S.r.l., Brusaporto (BG)) and the CertoClav MultiControl MC2- S09S273** steam sterilizer (CertoClav GmbH, Traun). “Dynamic-air-removal cycle”: 132°C (270°F) –...
-
Page 52: Storage
Hygiene and maintenance Storage Universal support > Store sterile goods dust-free and dry. > The shelf life of the sterile goods depends on the storage conditions and type of packaging. -
Page 53: Servicing
11. Servicing Periodic inspection Regular periodic inspection of the function and safety of the medical device is necessary and should be carried out at least once every three years, unless shorter intervals are prescribed by law. The periodic inspection covers the complete medical device and must only be performed by an authorized service partner. - Page 54 Servicing Repairs and returns In the event of operating malfunctions immediately contact an authorized W&H service partner. Repairs and maintenance work must only be undertaken by an authorized W&H service partner. Ensure that the medical device has been completely processed before returning it. Always return equipment in the original packaging.
-
Page 55: W&H Accessories And Spare Parts
12. W&H accessories and spare parts Use only original W&H accessories and spare parts or accessories approved by W&H. Suppliers: W&H partners (Link: https://www.wh.com) 04013500 07962790 07721800 Cassette Transportation case Universal support 04005900 06352200 3028100x Irrigant support Fuse (250 V - T1.6AH) EM-19 LC motor with electrical contactsand 1.8 m or 3.5 m cable... - Page 56 W&H accessories and spare parts 30285000 04653500 04719400 Foot control S-N2 Locator for foot control Irrigation tubing set 2.2 m 30264000 Foot control S-NW 04363600 06290600 30185000 Irrigation tubing set 2.2 m (6 pcs) Hose clips (5 pcs) EM-19 motor without 04364100 electrical contacts and 1.8 m cable...
- Page 57 W&H accessories and spare parts 08026120 08026150 ioDent® gateway mini ioDent® Wi-Fi dongle...
-
Page 58: Technical Data
13. Technical data Control unit SI-1023 SI-1015 SI-1010 Mains voltage: 230 V 120 V 100 V Permissible voltage fluctuation: 220 – 240 V 110 – 130 V 90 – 110 V Rated current: 0.3 – 0.8 A 0.3 – 1.6 A 0.3 –... - Page 59 Technical data Classification according to Paragraph 6 of the General Specifications for the Safety of Medical Electrical Device according to IEC 60601-1/ANSI/AAMI ES 60601-1 Class II medical electrical device (protective earth conductor used for functional earth connection only!) Pollution level: Overvoltage category: Altitude: up to 3,000 m above sea level...
-
Page 60: Data On Electromagnetic Compatibility According To Iec/En 60601-1-2
14. Data on electromagnetic compatibility according to IEC/EN 60601-1-2 Operating environment and EMC warning notes This medical device is neither life-sustaining nor coupled to the patient. It is suitable for operation both in domestic healthcare and in facilities used for medical purposes except rooms/areas, in which EMC interference of high-intensity may occur. - Page 61 Data on electromagnetic compatibility according to IEC/EN 60601-1-2 RF communication equipment Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 inches) to the medical device. Otherwise, degradation of the performance of this medical device could result.
- Page 62 Results of the electromagnetic tests Requirement Class / Test Level* Electromagnetic emissions Mains terminal disturbance voltage (Conducted Emissions) Group 1 CISPR 11/EN 55011 [150 kHz – 30 MHz] Class B Electromagnetic radiation disturbance (Radiated Emissions) Group 1 CISPR 11/EN 55011 [30 MHz – 1000 MHz] Class B Harmonic distortion IEC/EN 61000-3-2 Class A...
-
Page 63: Disposal
15. Disposal Ensure that the parts are not contaminated on disposal. Follow your local and country-specific laws, directives, standards and guidelines for disposal. > Medical device > Waste electrical equipment > Packaging... -
Page 64: W&H Course Certificate
W&H course certificate for the user The user has been trained to use the medical device correctly in accordance with the legal regulations (medical devices marketing regulations, medical devices act). Particular attention has been paid to the chapters on safety notes, start-up, operation, hygiene and maintenance, and service (regular inspections). Product name Serial number (SN) Manufacturer with address... - Page 65 W&H course certificate for the instructor The user has been trained to use the medical device correctly in accordance with the legal regulations (medical devices marketing regulations, medical devices act). Particular attention has been paid to the chapters on safety notes, start-up, operation, hygiene and maintenance, and service (regular inspections). Product name Serial number (SN) Manufacturer with address...
-
Page 67: Explanation Of Warranty Terms
Ex p la na ti on o f war ranty ter ms This W&H medical device has been manufactured with great care by highly qualified specialists. A wide variety of tests and controls guarantees faultless operation. Please note that claims under warranty can only be validated when all the directions in the Instructions for Use have been followed. -
Page 68: Authorized W&H Service Partners
Authorized W&H service partners Find your nearest authorized W&H service partner at http://wh.com Simply go to the menu option “Service” for full details. Or simply scan the QR code. - Page 70 Manufacturer W&H Dentalwerk Bürmoos GmbH Ignaz-Glaser-Straße 53, 5111 Bürmoos, Austria Form Nr. 50873 AAE t +43 6274 6236-0, f +43 6274 6236-55 Rev. 006 / 23.09.2022 office@wh.com wh.com Subject to alterations...







Need help?
Do you have a question about the implantmed PLUS SI-1010 and is the answer not in the manual?
Questions and answers