Table of Contents
Advertisement
Quick Links
Instructions for Use
ME PAD
The information in these Instructions for Use applies to the ME PAD. This information is subject
to change. Please contact Medical Econet GmbH. or its authorized representatives for
information on revisions.
Revision History
Edition 1
Publication Date: August 2011
Document No.: OPM-SP1- E-01
Published by: CU Medical Systems, Inc.
Printed in the Republic of Korea
Copyright
© 2011 Medical Econet GmbH
No part of these Instructions for Use may be reproduced without the permission of CU
Medical Systems, Inc.
Medical Device Directive
The ME PAD complies with the requirements of the Medical Device Directive 93/42/EEC and its
revisions.
Important:
Quick defibrillation is needed if sudden cardiac arrest occurs. Since the chance of success is
reduced by 7% to 10% for every minute that defibrillation is delayed, defibrillation must be
performed promptly.
1
Advertisement
Table of Contents
Troubleshooting

Summary of Contents for Medical Econet ME PAD
- Page 1 Instructions for Use ME PAD The information in these Instructions for Use applies to the ME PAD. This information is subject to change. Please contact Medical Econet GmbH. or its authorized representatives for information on revisions. Revision History Edition 1 Publication Date: August 2011 Document No.: OPM-SP1- E-01...
- Page 2 The ME PAD is manufactured by: CU Medical Systems, Inc. Dongwha Medical Instrument Complex 1647-1 Dongwha-ri, Munmak-eup, Wonju-si, Gangwon-do, 220-801 Republic of Korea Authorized EU Representative Medical Device Safety Service Schiffgraben 41, 30175 Hannover, Germany Contact Us For Orders & Inquiries...
- Page 3 ME PAD Public Access Defibrillator...
-
Page 4: Table Of Contents
DEVICE FEATURES ......................11 PREPARATION FOR USE ....................14 3.1 S TANDARD ACKAGE ONTENTS 3.2 S ME PAD ETTING UP THE HOW TO USE THE ME PAD ....................17 4.1 C HAIN OF URVIVAL 4.2 P REPARATION FOR EFIBRILLATION 4.3 D EFIBRILLATION IN DULT Step 1: Place pads on the patient.................. - Page 5 4.4 D EFIBRILLATION ROCEDURES IN EDIATRIC AFTER USING THE ME PAD .................... 28 5.1 M AINTENANCE FTER 5.2 S AVING AND RANSFERRING REATMENT 5.2.1 Device Usage ......................29 5.2.2 Transferring Treatment Data ................... 29 5.3 D EVICE ETTING 5.3.1 CPR Guide Setting ....................32 5.3.2...
- Page 6 B.1 S TANDARD CCESSORIES B.2 O PTIONAL CCESSORIES C . DESCRIPTION OF SYMBOLS ..................... 51 C.1 ME PAD D EFIBRILLATOR C.2 ME PAD P ACKAGING C.3 A CCESSORIES C.3.1 Disposable Battery Pack (CUSA1103BB, CUSA1103BS) ........... 53 C.3.2 Pads (CUA1007S, CUA1102S) ..................54 D .
-
Page 7: Introduction
NTRODUCTION These Instructions for Use contain information necessary for the correct use of this device. Please contact us regarding any questions or issues on the use of the device arising from information found in these Instructions for Use [Chapter 9: Device Service]. The company or its authorized distributor is not responsible for any injury incurred by the user or patient due to any apparent negligence or improper use by the user. -
Page 8: Overview
Overview Thank you for purchasing the ME PAD. This device can be effectively and safely used for a long period if you familiarize yourself with the instructions, warnings, precautions, and notices contained in these Instructions for Use prior to its use. -
Page 9: Introduction
The ME PAD is easy to use. It guides the you throughout a rescue operation using voice prompts and indicators (LED and graphical indicators). The ME PAD is small, light, highly portable, and battery powered. It is highly suitable for use in public, out-of-hospital settings. -
Page 10: Intended Users
1.3 Intended Users The ME PAD is intended for use in or out of the hospital by emergency care personnel or healthcare professionals or laypersons. The manufacturer recommends that users train on the use of the device. 1.4 Local Protocol Please contact your local health authority for information on the requirements of ownership and usage of defibrillators. -
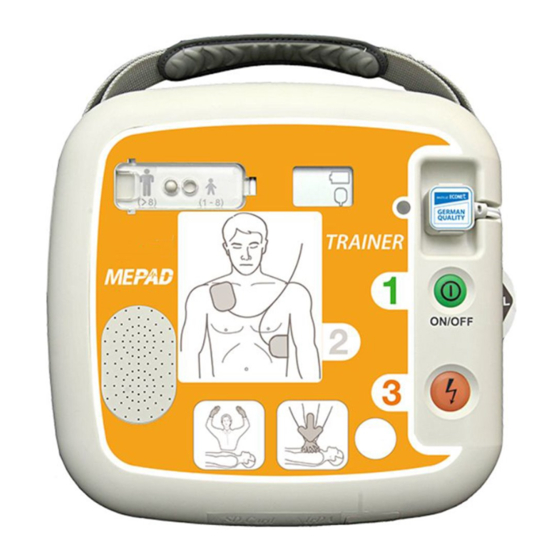
Page 11: Device Features
2. Device Features Adult/Pediatric Selection Switch Status LCD Defibrillator Pads Connector Pads Storage Compartment i-Button Do-Not-Touch-Patient Indicator CPR Detection Indicator Pad Connector Status Indicator Pad Position Indicators Power Button Adult/Pediatric Selection Shock Button Switch Cover IrDA Port Battery Pack SD Card Port... - Page 12 Turns the device on or off. (When the device is on, a green Power Button LED is lit) Reports device usage (the total hours of the last usage i-Button and number of shocks) checks the S/W version downloads events and ECG data via an IrDA and SD Card ...
- Page 13 Indicates performance of CPR on the patient. CPR Detection Indicator (The indicator is lit if CPR is performed, and flashes if CPR is not performed) Battery Pack The disposable power source of the device. IrDA Port Transmits and receives treatment data between the device and a personal computer.
-
Page 14: Preparation For Use
3. Preparation for Use 3.1 Standard Package Contents The following are the standard package contents of this device CU-SP1 Semi-automated External Defibrillator Instructions for Use 1 Battery Pack (Disposable) 1 Pack of Adult Pads (Disposable) Please contact the manufacturer for replacement supplies (refer to Appendix B: Parts and Accessories of these Instructions for Use). -
Page 15: Setting Up The Me Pad
Only parts and accessories recommended and approved by CU Medical Systems, Inc. must be used with the ME PAD. Using unapproved parts and accessories may compromise the safety and effectiveness of the ME PAD. Extra battery packs and pads are recommended. - Page 16 The use of accessories or cables other than those referred to in these Instructions for Use may increase electromagnetic radiation from the device or reduce the device’s electromagnetic immunity. Only accessories and cables that are authorized by the manufacturer should be used with the ME PAD.
-
Page 17: How To Use The Me Pad
4. How to Use the ME PAD 4.1 Chain of Survival If you think that you are witnessing someone go down in sudden cardiac arrest, perform the chain of actions recommended by the American Heart Association (AHA) in its Chain of Survival emergency response to sudden cardiac arrest. -
Page 18: Preparation For Defibrillation
• Set the switch to adult defibrillation mode as shown in the following picture Child victim (victim is under 25kb or 8 years old) If the pediatric pads are attached, the ME PAD automatically adjusts its defibrillation energy output for pediatric defibrillation regardless of the position of the Adult/Pediatric Selection Switch (i.e. - Page 19 8 years old. Ensure the slide key for Adult/Pediatric Mode is as shown on the bottom. You can switch the adult/pediatric selection switch before or after turning on the ME PAD. However, the defibrillation mode should be changed before placing the pads on the patient.
- Page 20 ③ Remove clothes from patient's chest. Time is essential for the cardiac arrest patient. Tear or cut clothes if removing them will take time. Dry the patient's skin such that pads can adhere well on the chest. Shave hair on the chest if necessary.
- Page 21 ⑥ Take pads out of the pads package. ⑦ Refer to the pictures on both pads. Adult Pads Pediatric Pads The adhesive material on the pads starts to dry out as soon as the package is opened. Use immediately after opening. Refer to Section 6.2: Maintenance of these Instructions for Use for procedures on how to check the expiration date of the pads and pads maintenance.
-
Page 22: Defibrillation In Adult Mode
4.3 Defibrillation in Adult Mode Step 1: Place pads on the patient. ① Remove pad 1 from the single liner and stick the pad to the patient’s upper chest as shown below. ② Remove pad 2 from the single liner, and stick the pad to the patient’s side torso as shown below. -
Page 23: Step 2: Press The Shock Button If Instructed
Step 2: Press the Shock Button if instructed. The device acquires and analyzes the patient's ECG immediately after being connected. The device will instruct you not to touch the patient by flashing the Do-Not-Touch-Patient Indicator and by issuing the voice prompt: “Do not touch the patient, analyzing heart rhythm”. After analyzing the ECG, the device will determine whether or not the patient needs defibrillation. - Page 24 If the patient does not need defibrillation, the device will do the following in sequence: • the device announces that the patient does not need a defibrillating shock and that you may touch the patient. • the CPR Mode Indicator is lit. •...
-
Page 25: Step 3: Perform Cpr
Step 3: Perform CPR. Perform CPR when the ME PAD instructs you to do so. By default, the CU-SP1 gives voice instruction for CPR during pause for CPR after a shock delivery. When voice instruction for CPR is needed outside of the default setting, press the flashing blue i-Button for at least 20 seconds. - Page 26 If you have not been trained in CPR, you should perform only chest compression or follow the instructions of the emergency medical services’ agent on the phone. If you are trained for CPR and able to perform artificial respiration, perform the chest compression along with artificial respiration.
-
Page 27: Defibrillation Procedures In Pediatric Mode
• When giving first aid during a pediatric cardiac arrest, ask others to call the emergency medical center and to bring the ME PAD while you are performing pediatric CPR. • When there is no one else around, perform CPR for 1 to 2 minutes, call emergency medical services, and then get the ME PAD. -
Page 28: After Using The Me Pad
5. After Using the ME PAD 5.1 Maintenance After Each Use • Check if the device for signs of damage and contamination. • If there is dirt contamination, see Section 6.2.3 on how to clean the device. • Run a battery insertion test. Refer to Section 8.1: Self-Diagnostic Test for the procedure. -
Page 29: Saving And Transferring Treatment Data
The recorded treatment data may be transferred to a personal computer (PC). This ME PAD keeps the data of the 5 most recent treatment operations and can save up to 3 hours of ECG data for each rescue operation. ECG data beyond 3 hours will not be recorded. - Page 30 into administrator mode with voice guide. ④ The device then gives you a summary (the total hours of the last device use and the number of defibrillation shocks delivered). ⑤ The voice guide gives the S/W version of the device. ⑥...
- Page 31 defibrillation shocks delivered). ④ The voice guide gives the S/W version of the device. ⑤ When instructed to transfer the treatment history, press the i-Button to transfer the data. If there is treatment data in the device’s internal memory: ① The voice guide reports the total number of individual treatment data recorded in the device. ②...
-
Page 32: Device Setting
5.3 Device Setting 5.3.1 CPR Guide Setting The default CPR setting on CU-SP1 is 5 cycles with 30 chest compressions and 2 breaths in accordance with the American Heart Association (AHA) 2010 CPR Guidelines. However, you may customize these. You can set the following: •... - Page 33 ⑧ Settings can then be changed in the following order: Number of Chest Compressions, Number of Artificial Respirations, Chest Compression rate, Pausing Time, and Detailed Guide Selection. Refer to Table 1: CPR Guide Setting Options below ⑨ When the setting is completed, the voice guide will give information regarding the set CPR guide, which may be saved or canceled.
- Page 34 If the Detailed Guide Selection is OFF and the Number of Artificial Respiration is set to 0, the ME PAD provides only chest compression guidance for 2 minutes. After 2 minutes, the ME PAD automatically reanalyzes the patient’s ECG...
-
Page 35: Maintenance
6. Maintenance 6.1 Device Storage Please refer to the precautions below when storing the Device in order to avoid device damage. Do not operate or store the device in conditions that are beyond the following. specified limits. • Storage Conditions The device is stored together with the defibrillator pads and the battery pack is inserted - ready to be used in an emergency Temperature: 0℃... -
Page 36: Maintenance
Refer to Section 8.1: Self-Diagnostic Test for more information. Inspect the ME PAD daily to ensure that it is always ready for an emergency. Check the current status of the device, battery, and pads as displayed on the Status LCD. - Page 37 2. Insert a new battery pack in the direction of the arrow with the label facing upward as shown in the figure below. 3. Push the battery pack until you hear it click into place. Battery Pack Precautions • Do not subject the battery pack to serious physical impact. •...
-
Page 38: Replacing The Pads
• Check the pads package for damage. • Check the cable outside the packaging pouch for possible defects. • Only pads provided by the manufacturer should be used with the ME PAD. Replacing Pads 1. Check the expiration date of the pads. Refer to the figure below for checking the expiration date. -
Page 39: Cleaning The Me Pad
IrDA port may be damaged. Do not use a detergent containing abrasive ingredients. Do not sterilize the ME PAD. 7. Disposal Dispose of ME PAD and its accessories in accordance with local regulations. -
Page 40: Troubleshooting
8. Troubleshooting 8.1 Self-Tests The following table lists the self-tests done by the device. Self-Test Type Description Battery Insertion Runs when the battery pack is inserted into the device. Test Perform this test: • Before the device is deployed • After each use •... - Page 41 Self-Test Type Description Power ON Test The device performs a self-diagnostic test when the Power Button is pressed Run-time Test The device monitors itself in real-time during its operation. Periodic This device performs self-diagnostic tests daily, weekly and monthly. The Self-Diagnostic periodic self-test checks important features of the device such as the Test...
-
Page 42: Device Status
8.2 Device Status The status of the device is indicated by the following symbols: Indicator Description Note Status LCD The device is functioning normally. Device Operation Status LCD The device has an error. Device Operation Status LCD The battery is fully charged. Battery Level Indicator Status LCD Less than half battery power remains. -
Page 43: Troubleshooting
8.3 Troubleshooting The device informs you of its current status or of problems via status indicators, beeps, and/or voice instruction. Refer to the following for details: 8.3.1 Troubleshooting While the Device is Operating Symptom/Voice Instruction Cause Resolution Immediately replace the Status LCD An error has occurred in defibrillator and perform... -
Page 44: Troubleshooting While The Device Is Not Operating
Although an electric shock Deliver an electric shock by Voice Prompt : is needed, the Shock pressing the Shock Button ” Shock button was not pressed” Button was not pressed with the next voice within 15 seconds. instruction. If the problem cannot be solved during an emergency, you should follow the following steps: ①... -
Page 45: Device Service
9. Device Service Device Warranty Device Name Model Name Purchase Name Serial No. Distributor Person in Charge This device is warranted by CU Medical Systems, Inc. against defects in materials and workmanship for five full years from the date of original purchase. During the warranty period, we will repair or, at our option, replace at no charge a device that proves to be defective, provided you return the device, shipping prepaid, to us or to our authorized representative. - Page 46 The ME PAD must be serviced only by authorized personnel. The ME PAD will be serviced free of charge during the warranty period. After the warranty period, the cost of material and service shall be shouldered by the user.
-
Page 47: Appendix
Appendix A . Rescue Protocol Power ON Voice Prompt: “Call Emergency Medical Services, now.” Voice Prompt: “Plug the pads connector into the device.” Pads Connected? Voice Prompt: “Adult mode” or “Pediatric mode” PADS ON Voice Prompt: “Follow the voice prompt calmly.” Voice Prompt: “Remove all clothing from chest and stomach. - Page 48 A’ Voice Prompt: “Do not touch the patient.” “Analyzing heart rhythm.” i-PAD CU-SP1 detect/decide: ECG Shockable? Voice Prompt: “Shock advised.” “Stand clear.” Voice Prompt: “No shock advised.” Charging Voice Prompt: “Charging now.” Complete? Voice Prompt: “Press the flashing orange button, now.” “Deliver shock, now.”...
- Page 49 B’ Voice Prompt: “Press the flashing blue i-button for CPR voice prompt.” Within 20 seconds, i-button is:? Voice Prompt: Re-analyzing heart rhythm in 2 minutes.” Voice Prompt: “Place the heel of one hand on the center of the chest, between the nipples.” Voice Prompt: “Re-analyzing heart rhythm in 1 minute.”...
-
Page 50: Parts And Accessories
B . Parts and Accessories To order replacement parts and accessories, cite the part and ordering numbers given in the following table. B.1 Standard Accessories Name Part Number Ordering Number Adult Pads (disposable) CUA1007S SP1-OA04 Disposable Battery Pack(Long-life) CUSA1103BB SP1-OA03 Instructions for Use SP1-OPM-E-01 B.2 Optional Accessories... -
Page 51: Description Of Symbols
C . Description of Symbols C.1 ME PAD Defibrillator Symbol Description Power ON/OFF button i-Button SHOCK button Adult / Pediatric Selection Switch Do-Not-Touch-Patient Indicator CPR Detection Indicator BF type, defibrillation-proof equipment Attention: Refer to accompanying documents. CE Mark; meets the requirements of the European Medical Device Directive 93/42/EEC and its revisions. -
Page 52: Me Pad Packaging
C.2 ME PAD Packaging Symbol Description Stack up to 6 cartons high only This side up Keep dry Fragile; breakable Use no hooks Storage Temperature limits: 0℃ to 43℃ (32℉ to 109℉ ) CE Mark; meets the requirements of the European Medical Device Directive 93/42/EEC and its revisions. -
Page 53: Accessories
C.3 Accessories C.3.1 Disposable Battery Pack (CUSA1103BB, CUSA1103BS) Symbol Description Lithium Manganese Dioxide battery Lot Number Expiration date and Install by date Do not mutilate the battery or open the battery case Do not expose the battery to high heat or open flames. Do not incinerate the battery. -
Page 54: Pads (Cua1007S, Cua1102S)
C.3.2 Pads (CUA1007S, CUA1102S) Symbol Description Temperature limits: 0℃ to 43℃ (32℉ to 109℉ ) Lot number Expiration date Order reference number Single use only; do not reuse Do not fold or bend. Contains no latex Expiration Date and Lot number sticker Attention: Refer to accompanying documents CE Mark;... -
Page 55: Glossary
D . Glossary 1 CPR 1 CPR consists of 5 cycles. (When the device is set to 5 cycles as default) Refers to 30 chest compressions followed by 2 breaths during 1 Cycle CPR. (When the device is set to the default setting [30:2]) If you specify the number of compression and number of breath, the cycle is performed in accordance with the specified protocol. - Page 56 An abnormal heart rhythm. Arrhythmia Battery Pack A disposable battery that supplies power to the ME PAD. Cardiac Arrest A patient with cardiac arrest symptoms. This device should be used for the patient with the following symptoms: No Patient response, no movement and no normal breathing.
- Page 57 defibrillation via an electric shock. Error A status in which the device does not properly operate. Refer to [Section 8.3: Troubleshooting] for more information. Refers to an irregularity of the heart causing ineffective Fibrillation circulation. Ventricular fibrillation is accompanied with an acute cardiac arrest.
- Page 58 ME PAD. PC S/W CU Expert PC software used to modify the settings of the ME PAD and (CU-EX1) to manage treatment data. Refer to the appendix on accessories if you want to purchase this software. Pediatric The child in these Instructions for Use is defined as a person who is older than 1 year and younger than 8 years as well as lighter than 25 kg.
- Page 59 The button that you must press to deliver an electric shock to a cardiac arrest patient. Standby Mode The mode of the ME PAD when the Power Button is OFF but the battery pack is inserted. If is shown on the Status LCD while the device is in standby mode, the device is ready to be used as needed in an emergency).
-
Page 60: Device Specifications
E . Device Specifications Model Name: ME PAD Physical Category Nominal Specifications Dimensions 260㎜ x 256㎜ x 69.5㎜ (Width x Length x Height) Weight 2.4㎏ (Including the battery pack and pads) Environmental Category Nominal Specifications Operational Status (The device is in emergency use) Temperature: 0℃... - Page 61 When the impedance of the patient is out of the range of defibrillation (25 Ω ~ 175 Ω) Shock Delivery Shock is delivered if the SHOCK button is pressed while the ME PAD is armed. Adult pads in the anterior-anterior position Shock Delivery ...
- Page 62 time(ms) Biphasic Truncated Exponential Type. The shock waveform profile is automatically compensated for the patient’s transthoracic impedance. A = first phase duration B = second phase duration0 C = interphase duration D = peak current Output Waveform for Adult (150 Joules) Patient First Phase Second Phase...
- Page 63 ECG Acquisition Category Nominal Specifications Acquired ECG Lead Lead II Frequency Response 1 Hz to 30 Hz ECG Analysis System Category Nominal Specifications Determines the impedance of the patient and evaluates the ECG of the Function patient to determine whether it is shockable or non shockable 25Ω...
- Page 64 ECG Analysis System - ECG Database Test 90% One Minimum Test Performan Shock No Shock Observed Sided Lower Rhythm Rhythms test sample sample ce goal Decision Decision Performance Confidence Class size size Limit 97.26% >90% Coarse VF (213/219) sensitivity sensitivity 81.02% >75% Fast VT...
- Page 65 Blue i-Button: Flashes when guiding CPR, transferring the treatment history and setting the CPR mode. Red i-Button: Flashes when an error occurs. Plays back voice instructions. The ME PAD analyzes the ambient noise level Speaker during a treatment operation. If ambient noise level is high, it automatically increases the voice instructions volume so that you can hear them clearly.
- Page 66 Self-Diagnostic Test Power On Self-Test, Run-time Self-Test Auto Daily, Weekly, and Monthly Self-Test Battery Pack Insertion Test (done when the user inserts the battery pack into Manual the battery pack compartment of the device) Disposable Battery Pack Category Nominal Specifications 12V DC, 2.8Ah LiMnO , Disposable: Standard...
- Page 67 Adult Defibrillation Pads (CUA1007S) Category Nominal Specifications Type Adult Electrode Area 120 cm Cable Length Total 120 cm (Inside the pouch: 95 cm, Outside the pouch: 25 cm) Shelf life At least 36 months from the date of manufacture Pediatric Defibrillation Pads (CUA1102S) Category Nominal Specifications Type...
-
Page 68: Electromagnetic Compatibility
Guidance and manufacturer’s declaration – electromagnetic emissions The ME PAD is intended for use in the electromagnetic environment specified below. The customer or the user of the ME PAD should assure that it is used in such an environment. Emissions Test... - Page 69 Guidance and manufacturer’s declaration – electromagnetic immunity The ME PAD is intended for use in the electromagnetic environment specified below. The customer or the user of the ME PAD should assure that it is used in such an environment. IEC 60601-1 test...
- Page 70 The ME PAD is intended for use in the electromagnetic environment specified below. The customer or the user of the ME PAD should assure that it is used in such an environment. Immunity IEC 60601 Test Complia Electromagnetic environment - guidance...
- Page 71 RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the ME PAD is used exceeds the applicable RF compliance level above, the ME PAD should be observed to verify normal operation.
- Page 72 ME PAD The ME PAD is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the ME PAD can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the ME PAD as recommended below, according to the maximum output power of the communications equipment.
Need help?
Do you have a question about the ME PAD and is the answer not in the manual?
Questions and answers