Pari LC PLUS Instructions For Use Manual
Hide thumbs
Also See for LC PLUS:
- User manual ,
- Instructions for use manual (21 pages) ,
- Instructions (2 pages)
Table of Contents
Advertisement
Quick Links
Instructions for use
PARI LC PLUS
PARI LC STAR
PARI LC PLUS
Model: PARI LC PLUS (Type 022)
Nebulisers for PARI inhalation systems
Important: Read these instructions carefully before using
the product for the first time. Follow all instructions and
safety instructions!
Keep the instructions in a safe place.
®
®
®
Baby
en
Advertisement
Table of Contents

Summary of Contents for Pari LC PLUS
- Page 1 ® PARI LC STAR ® PARI LC PLUS Baby Model: PARI LC PLUS (Type 022) Nebulisers for PARI inhalation systems Important: Read these instructions carefully before using the product for the first time. Follow all instructions and safety instructions! Keep the instructions in a safe place.
- Page 3 – 3 – Identification, validity, version These instructions for use are valid for PARI nebulisers (Type 022) in the following countries: Countries outside the EU Version of these instructions for use: Version F – 2020-02, Approved version dated 2019-11-15 Information as of: 2019-11 The current version of the instructions for use can be down- loaded from the internet as a PDF file: www.pari.com (on the respective product page)
- Page 4 – 4 – Contact For all product information and in the event of defects or ques- tions about usage, please contact our Service Center: Tel.: +49 (0)8151-279 220 (international) +49 (0)8151-279 279 (German-speaking) E-Mail: info@pari.de...
-
Page 5: Table Of Contents
– 5 – TABLE OF CONTENTS IMPORTANT INFORMATION.......... Intended purpose ............. Indication ................Contraindications.............. Safety instructions ............PRODUCT DESCRIPTION ..........Components ..............Product variants ............... Product combinations ............Description of function ............Material information ............Operating life ..............USE .................. Preparing for treatment ............ - Page 6 – 6 – MISCELLANEOUS ............Disposal................Labelling ................APPENDIX: Reprocessing in professional environ- ments for use with several patients ......Nebuliser ................Connection tubing ............
-
Page 7: Important Information
The nebuliser must only be used with a PARI compressor. For reasons of hygiene, this PARI product must only be used in a home environment by a single patient. The inhalation system must only be used by individuals who understand the contents of the instructions for use and are able to operate the inhalation system safely. -
Page 8: Indication
The user must follow these in order to guarantee safe op- eration of this PARI product. This PARI product must only be used as described in these in- structions for use. The instructions for use of the compressor and accessories used and the information for use of the inhalation solution used must also be followed. - Page 9 – 9 – Hazard due to small parts which can be swallowed The product contains small parts. Small parts can block the air- ways and lead to a choking hazard. Keep all components of the product out of the reach of babies and infants at all times. Hygiene Observe the following hygiene instructions: –...
- Page 10 – 10 – Identifying and classifying warning instructions Safety-critical warnings are categorised according to the follow- ing hazard levels in these instructions for use: DANGER DANGER indicates a hazardous situation which will lead to severe injuries or death if it is not avoided. WARNING WARNING indicates a hazardous situation which can lead to severe injuries or death if it is not avoided.
-
Page 11: Product Description
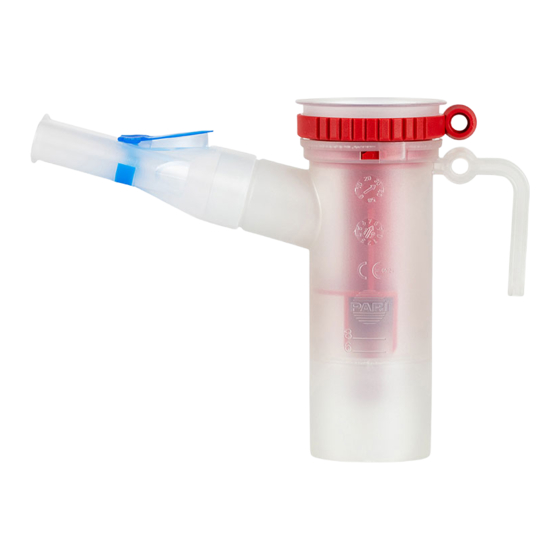
– 11 – PRODUCT DESCRIPTION 2.1 Components The following components are included in the package: (1) Nebuliser (1a) Inspiratory valve (1b) Nozzle insert (1c) Nebuliser lower part (1d) LC interrupter (1e) Mouthpiece (1f) Connection tubing (2) Air filter for compressor 3) Not included with all product variants. -
Page 12: Product Variants
Not all products are available in all countries. 2.3 Product combinations The PARI nebuliser can be operated with all PARI compressors. It can be used in combination with a range of PARI accessories. 2.4 Description of function The PARI nebuliser is part of a PARI inhalation system. -
Page 13: Material Information
– 13 – The LC interrupter makes it possible to interrupt aerosol gener- ation while the patient breathes out, thereby optimising medica- tion use. 2.5 Material information The individual product components are made from the follow- ing materials: Product component Material Inspiratory valve Polypropylene, silicone Nozzle insert... -
Page 14: Use
People who assist others in carrying out the therapy must ensure that all of the steps described below are carried out correctly. If the nebuliser is to be operated via a PARI CENTRAL on a central medical gas supply, the instructions for use of the PARI CENTRAL must be followed. - Page 15 – 15 – • Attach the connection tubing to the nebuliser. • Attach the LC interrupter to the nebuliser. • Insert the connection tubing in the air inlet on the side of the LC interrupter. Using the mouthpiece • Fit the mouthpiece onto the nebuliser.
- Page 16 – 16 – Filling the nebuliser • Insert the nebuliser in the holder on the compressor in- tended for this purpose. • If applicable, detach the inspiratory valve from the nebuliser. • Pour the required quantity of inhalation solution into the top of the nebuliser.
-
Page 17: Performing Treatment
– 17 – 3.2 Performing treatment All of the safety instructions in these instructions for use must have been read and understood before any treatment is carried out. Always hold the nebuliser upright during treatment. Proceed as follows in order to carry out the treatment: •... - Page 18 – 18 – • Switch the compressor on. DANGER! Danger of death by electrocution in the case of device fault! Switch the compressor off and discon- nect the power plug from the mains socket immediately if a fault is suspected (e.g., follow- ing a fall or if there is a smell of burning plastic).
-
Page 19: Ending The Treatment
– 19 – Using the LC interrupter If the LC interrupter is attached, aerosol is not generated until the interrupter button is pressed. Proceed as follows to inhale and to interrupt aerosol generation when breathing out: • Press the interrupter button to generate aerosol. -
Page 20: Reprocessing
– 20 – REPROCESSING The product components must be cleaned thoroughly immedi- ately after each use and disinfected at least once a day. If the nebuliser is used in professional environments, follow the information on reprocessing included in the appendix at the end of these instructions for use. -
Page 21: Disinfecting
– 21 – 4.3 Disinfecting After cleaning, disinfect all of the disassembled parts (only parts that have been cleaned can be disinfected effectively). The recommended disinfection procedures are described be- low. Descriptions of other validated disinfection procedures are available from the manufacturer or dealer upon request. The connection tube cannot be cleaned or disinfected. -
Page 22: Care Of The Connection Tube
– 22 – Using a standard thermal disinfector for baby bottles (not a microwave oven) CAUTION Risk of infection due to inadequate disinfection Inadequate disinfection encourages the growth of bacteria and thus increases the risk of infection. • Make sure that the disinfector is clean and operating prop- erly before every disinfection process. -
Page 23: Storage
Weight 27.5 g to 29.5 g Operating gases Minimum compressor flow 3.0 l/min. Minimum operating pressure 0.5 bar / 50 kPa Maximum compressor flow 6.0 l/min. Maximum operating pressure 2.0 bar / 200 kPa Minimum fill volume 2 ml Maximum fill volume 8 ml; PARI LC STAR: 6 ml 5) Without mouthpiece; unfilled. -
Page 24: Aerosol Data According To Iso 27427
[% > 5 µm] Aerosol output [ml] 0.44 0.49 0.34 Aerosol output rate 0.05 0.07 0.08 [ml/min] Residual volume 0.99 0.94 0.99 [ml] (gravimetric) 6) Operation with PARI COMPACT2 compressor (Type 152). 7) MMAD = Mass Median Aerodynamic Diameter 8) GSD = Geometric Standard Deviation... - Page 25 – 25 – Minimum com- Nominal com- Maximum com- pressor flow pressor flow pressor flow Nozzle insert (red) (3 l/min – (4 l/min – (6 l/min – 0.6 bar) 1.2 bar) 1.9 bar) Percentage of fill volume emitted per minute [%/min] Minimum com- Nominal com- Maximum com- Nozzle insert pressor flow pressor flow...
-
Page 26: Miscellaneous
– 26 – MISCELLANEOUS 7.1 Disposal All product components can be disposed of with domestic waste unless this is prohibited by the disposal regulations pre- vailing in the respective member countries. 7.2 Labelling The following symbols can be found on the product and/or the packaging: Legal manufacturer The product satisfies the basic requirements as set forth... -
Page 27: Appendix: Reprocessing In Professional Environments For Use With Several Patients
– 27 – APPENDIX: Reprocessing in professional environments for use with several patients Nebuliser The following overview of the processing steps in professional environments applies to the following products: – Nebuliser – LC interrupter 1. Preparation Disassemble the product [see: Preparation, page 20]. Check: –... - Page 28 – 28 – 2. Cleaning and disinfection ® Manual pH-neutral, enzymatic cleaner, e.g., Korsolex ® cleaning: Endo Cleaner (Bode) or Bodedex forte (Bode) Use: In accordance with manufacturer information, if dirt is visible use a brush if necessary. Manual With aldehyde-containing instrument disinfectant, ®...
-
Page 29: Connection Tubing
– 29 – 3. Steam sterilisation Equipment: – Steam steriliser (preferably with fractionated pre-vacuum) in accordance with DIN EN 285 or DIN EN 13060 (Type B) – Sterile barrier system in accordance with DIN EN 11607 Temperature / Duration: 134 °C for at least 3 min. 4. Visual inspection & storage Check: Inspect all individual parts. - Page 30 – 30 – ® Mechanical Alkaline cleaning agent, e.g., neodisher cleaning with MediClean forte (Dr. Weigert) in conjunction with ® disinfection: neutralising agent, e.g., neodisher Z (Dr. Weigert) Equipment: – Cleaning device and disinfector in conform- ance with DIN EN ISO 15883, e.g., RDG G7836 CD (Miele) –...
- Page 31 – 31 –...
- Page 32 PARI GmbH Spezialisten für effektive Inhalation Moosstraße 3 82319 Starnberg • GERMANY info@pari.de • www.pari.com...















Need help?
Do you have a question about the LC PLUS and is the answer not in the manual?
Questions and answers