Advertisement
Table of Contents
- 1 Indications for Use
- 2 System Description
- 3 Safety Features
- 4 System Hardware
- 5 Warnings, Precautions, and Adverse Reactions
- 6 Front Panel Controls
- 7 Display Panel
- 8 Connection Icons
- 9 Rear Panel Connections
- 10 Technical Specifications
- 11 Power Specifications
- 12 Separation Distances
- Download this manual
Ampere™ Generator
WARNING:
The use of this device in conjunction with a radio frequency ablation
catheter, as a part of the diagnosis and treatment of cardiac arrhythmias,
may pose an increased risk of adverse events such as cardiac perforation,
myocardial infarction, air embolism, and hematoma requiring surgical
repair and/or blood transfusion.
St. Jude Medical
One St. Jude Medical Drive
St. Paul, MN 55117 USA
651-756-6985 / 800-374-8038
651-647-9464 / 800-374-2505
EC REP
St. Jude Medical Coordination Center BVBA
The Corporate Village
Da Vincilaan 11 Box F1
1935 Zaventem Belgium
+32 2 774 68 11
+32 2 772 83 84
afcustomerservice@sjm.com
www.sjm.com
Australian Sponsor:
St. Jude Medical
Australia Pty Limited
17 Orion Rd.,
Lane Cove NSW 2066
AUSTRALIA
+61 2 9936 1200
I
NSTRUCTIONS FOR
I
NTERNATIONAL
Software version 1.0
© Copyright 2013
St. Jude Medical
Unless otherwise noted, ™ indicates that the name is a
trademark of, or licensed to, St. Jude Medical or one of its
subsidiaries. ST. JUDE MEDICAL and the nine-squares
symbol are trademarks and services marks of St. Jude
Medical, Inc. and its related companies. © 2013 St. Jude
Medical, Inc. All Rights Reserved.
Rev. A 100081509-en
1
U
SE
E
DITION
2013-08
Advertisement
Table of Contents

Summary of Contents for St. Jude Medical Ampere Generator
- Page 1 Unless otherwise noted, ™ indicates that the name is a may pose an increased risk of adverse events such as cardiac perforation, trademark of, or licensed to, St. Jude Medical or one of its myocardial infarction, air embolism, and hematoma requiring surgical subsidiaries.
- Page 2 Ampere™ Generator Instructions for Use International Edition Rev. A 100081509-en Symbol Description Symbol Description Weight Do not use if package is damaged. Fragile Package contains 1 item. Keep dry Defibrillator-proof type CF applied part Manufacturer Defibrillator-proof type BF applied part Date of Manufacture Power switch Use by date...
- Page 3 Ampere™ Generator Instructions for Use International Edition Rev. A 100081509-en Symbol Description Symbol Description Warning General Serious injury can occur if care is not Ablate taken during use of the system. Caution Menu Notified body CE Mark Preset Authorized representative in the EC REP Up Button European community...
- Page 4 Ampere™ Generator Instructions for Use International Edition Rev. A 100081509-en Symbol Description Rated Current Footswitch Generator Non-ionizing electromagnetic radiation. Cables Cords Package Contents IP68 Footswitch enclosure is dust tight and protected against submersion in water. Neutral Electrode (Floating Ground)
-
Page 5: Indications For Use
Ampere™ Generator Instructions for Use International Edition Rev. A 100081509-en Introduction Preface The use of all components and accessories of the Ampere™ Generator is described in this manual. The ablation catheter description can be found in a separate instruction for use manual available with the catheter. This manual provides a description of the Ampere™ Generator, its controls and displays, and a sequence for its operation. -
Page 6: System Description
Additional accessories to the generator include an optional footswitch the operator can use to turn on or off RF delivery. When connected with a compatible St. Jude Medical Cool Point™ irrigation pump, the Ampere™ Generator provides additional pump control options for use with an irrigated ablation catheter. -
Page 7: Safety Features
Ampere™ Generator Instructions for Use International Edition Rev. A 100081509-en Customizable Information Displays Various additional options are also available to the user from the menu, including statistics on power, temperature, and impedance from previous ablation applications, display of additional information during ablation applications, and language, audio volume, and screen brightness control. -
Page 8: Warnings, Precautions, And Adverse Reactions
Ampere™ Generator Instructions for Use International Edition Rev. A 100081509-en Warnings, Precautions, and Adverse Reactions Definitions WARNING: A warning contains instructions for avoiding hazardous situations that could cause significant injury to a patient or operator. CAUTION: A caution contains instructions for avoiding hazards that could adversely affect system components or system performance. - Page 9 Ampere™ Generator Instructions for Use International Edition Rev. A 100081509-en Precautions CAUTION: While using an irrigated catheter, the temperature displayed on the Ampere™ Generator is the temperature of the cooled electrode at the tip of the catheter—not the tissue temperature. CAUTION: Always verify that the tubing and catheter have been properly cleared of air prior to inserting the catheter into the vasculature since entrapped air bubbles may cause emboli.
- Page 10 Ampere™ Generator Instructions for Use International Edition Rev. A 100081509-en CAUTION: This equipment generates, uses, and can radiate radio-frequency energy and, if not installed and used in accordance with the instructions, may cause harmful interference to other devices in the vicinity. However, there is no guarantee that interference will not occur in a particular installation.
-
Page 11: Front Panel Controls
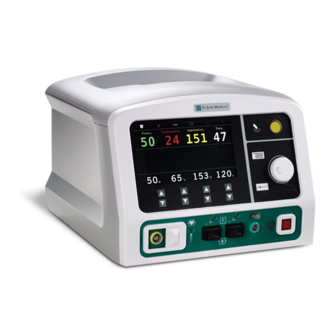
Ampere™ Generator Instructions for Use International Edition Rev. A 100081509-en Display, Controls, and Connections Front Panel Controls Figure 1. Front Panel Controls Callout Name Description RF energy delivery • Press to begin delivery of RF energy. Press again to stop RF energy delivery. Button Menu Button •... -
Page 12: Display Panel
Ampere™ Generator Instructions for Use International Edition Rev. A 100081509-en Display Panel Therapy data display, parameter adjustments, and generator setup are made through the display panel. Figure 2. Display Panel Callout Name Description Real-time Display • Displays Power, Temperature, Impedance, and Time values as therapy is being delivered. -
Page 13: Connection Icons
Ampere™ Generator Instructions for Use International Edition Rev. A 100081509-en Connection Icons The Connection Icons indicate the state of connected accessories: Figure 3. Connection Icons Icon Name State Description TempGuard™ Gray • The TempGuard™ feature is not enabled. Feature White •... -
Page 14: Rear Panel Connections
Ampere™ Generator Instructions for Use International Edition Rev. A 100081509-en Rear Panel Connections Figure 4. Rear Panel Connections Callout Name Description Foot Switch Connector Connects to the foot switch. Equipotential Ground Connects the Ampere™ Generator to the hospital equalization connection Connector point. -
Page 15: Technical Specifications
Ampere™ Generator Instructions for Use International Edition Rev. A 100081509-en Technical Specifications Specifications Power Specifications Supply Voltage 100-240VAC, 50/60 Hz Current Rating 2.4 A typical at 115 VAC, full load 1.2 A typical at 230 VAC, full load Fuse Rating F1 &... - Page 16 Ampere™ Generator Instructions for Use International Edition Rev. A 100081509-en Physical Characteristics Operating Modes Temperature Control Mode Power Control Mode Input/Output 22 pin socket for the catheter Socket for the footswitch Serial interface (DB9 EP Recording System interface) Serial interface (DB15 Pump interface) Fiber Optic Connectors (Remote Control Interface) Dimensions Generator: 266.7mm H x 360.68mm W x 363.22mm D...
- Page 17 Ampere™ Generator Instructions for Use International Edition Rev. A 100081509-en Electromagnetic Immunity Declaration I The Ampere ™ Generator is intended for use in the electromagnetic environment specified below. The customer or the user of the Ampere ™ Generator should assure that it is used in such an environment. Immunity Test IEC 60601 Test Level Compliance Level...
- Page 18 Ampere™ Generator Instructions for Use International Edition Rev. A 100081509-en Electromagnetic Immunity Declaration II The EnSite ™ Velocity ™ Cardiac Mapping System Amplifier and Workstation are intended for use in the electromagnetic environment specified below. The customer or the user of the EnSite™ Velocity™ Cardiac Mapping System Amplifier / Workstation should assure that it is used in such an environment.
-
Page 19: Separation Distances
Ampere™ Generator Instructions for Use International Edition Rev. A 100081509-en Separation Distances Recommended separation distances between portable and mobile RF communications equipment and the Ampere ™ Generator The Ampere ™ Generator is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the Ampere ™...












Need help?
Do you have a question about the Ampere Generator and is the answer not in the manual?
Questions and answers