Table of Contents
Advertisement
Quick Links
Advertisement
Table of Contents

Summary of Contents for Moller Medical Liposat power
- Page 1 ® Liposat power...
- Page 2 IMPORTANT READ CAREFULLY BEFORE USE KEEP THESE INSTRUCTIONS FOR FUTURE CONSULTATION...
-
Page 3: Manufacturer
® Liposat power Manufacturer Manufacturer Möller Medical GmbH Wasserkuppenstraße 29-31 36043 Fulda, Germany Tel. +49 (0) 661 / 94 19 5 - 0 Fax +49 (0) 661 / 94 19 5 - 850 http://www.moeller-medical.com E-mail: info@moeller-medical.com Page 3 of 36... -
Page 4: Table Of Contents
® Liposat power ® Liposat power configuration Table of contents Manufacturer ......................3 Table of contents ....................4 Product description ....................6 Intended use (intended purpose) ..................6 Essential performance feature ................... 6 Manufacturer ........................6 Product overview ....................... 7 ®... - Page 5 ® Liposat power Table of contents Alarms and service personnel ................24 Alarm conditions ......................24 Workplaces of the service personnel ................24 Assistance with problems ................. 25 Service station ....................27 Cleaning and care ....................28 Cleaning and disinfection ....................28 Maintenance ........................
-
Page 6: Product Description
® Liposat power ® Liposat power configuration Product description Any use of the product assumes precise knowledge of the application and attention to these instructions for use. This set of instructions does not replace professional instruction to the user by a certified medical device consultant. The product should be used only by persons who have the required training, knowledge and experience (§2 Section 2 MPBetreibV). -
Page 7: Product Overview
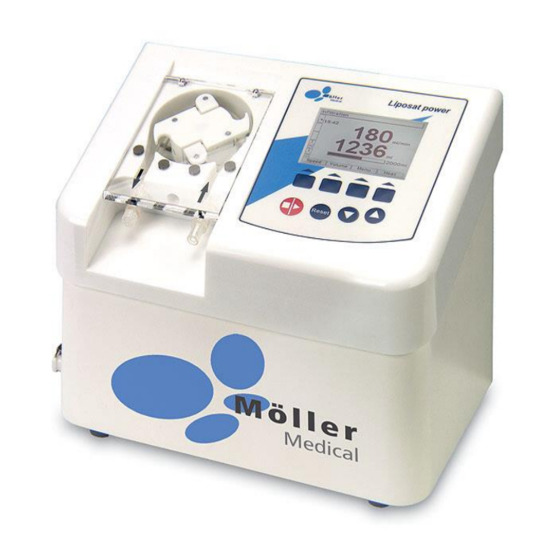
® Liposat power Product description Product overview Page 7 of 36... -
Page 8: Using The Liposat Power
® Liposat power ® Liposat power configuration ® Using the Liposat power The device is turned on with the ON – OFF switch on the left side of the device. The device then performs a self-test. The software version, the device number and the manufacturer are shown on the display. - Page 9 ® Liposat power Product description Example of infiltration application Adjusted flow rate = 180 ml/min Adjusted infiltration volume = 2000 ml After the pump was started, 180 ml/min will be infiltrated for 11.1 minutes (infiltration time). All necessary information is shown on the display. 1 –...
- Page 10 ® Liposat power ® Liposat power configuration Before usage, inspect the original packed tube set for shelf life and damages. Do not use expired, damaged, dirty or wet tube sets. Load the pump tube segment into the pump. The pump tube segment can be found between the adapters, which are intended to be attached into the adapter receptacle of the pump.
-
Page 11: Repeat Technical Safety Monitoring
® Liposat power Product description Accessories may be attached to the Liposat ® power. One or two heating dishes serve to maintain the temperature of the pre-heated containers of saline solution. The heating dishes will be turned on and off by using the soft key button Heating (8). Press the button Heating to enter the settings menu. -
Page 12: Technical Data
® Liposat power ® Liposat power configuration Technical data Article number 00002274 Dimensions of the device: (WxHxD) 270 mm x 220 mm x 180 mm Weight: approx. 10 kg Minimum operating life: 8 years Electrical connection: 100 – 240 VAC (alternating current) Mains voltage: 50 –... -
Page 13: General Safety Instructions
® Liposat power General safety instructions General safety instructions Obligations to the operator The operator takes responsibility for the proper operation of the medical device. The Ordinance on operation places a number of obligations on the user and responsibility as part of his activity in using medical devices. - Page 14 ® Liposat power ® Liposat power configuration The sodium chloride package must always lie with its surface solidly on the temperature sensor. The user must decide whether monitoring the body temperature of the patient is necessary and at what intervals that should occur in order to avoid any medical risks (hypothermia, hyperthermia, etc.).
-
Page 15: Explanation Of The Safety Symbols That Are Used
® Liposat power General safety instructions Explanation of the safety symbols that are used Important notices are specially marked off in this instructions for use. These notices are a condition for preventing dangers to the patient and the service personnel as well as to avoid damage to the product or functional disturbances. - Page 16 ® Liposat power ® Liposat power configuration Serial number Catalog number Batch code Use by YYYY-MM-DD Sterilised using ethylene oxide Do not re-use Do not resterilise Manufacturer Do not use if package is damaged Contains or presence of phthalates Stacking limit, do not store more than 3 packs high Attention: Under US Federal law, this device may be only sold to a physician or ordered by a physician.
-
Page 17: One-Time Use
® Liposat power General safety instructions One-time use Reuse of one-time use articles carries the potential risk of infection for the patient or the user. Contaminated articles can lead to injuries, illness or death of the patient. Cleaning, disinfection and sterilisation can damage important material characteristics and product parameters in a way that leads to the breakdown of the product. -
Page 18: Transport
® Liposat power ® Liposat power configuration Transport The following safety points must absolutely be followed when transporting the device. Damage to the device and other material damage can thereby be avoided. Absolutely follow the transport instructions on the package. ... -
Page 19: Assembly And Start-Up
® Liposat power Assembly and start-up Assembly and start-up Make sure that the package has been delivered to you undamaged. Immediately report any transportation damage to the shipper. Unpacking the device and checking the content of the delivery The delivery of the Liposat ®... -
Page 20: Suitable Operating Environments
® Liposat power ® Liposat power configuration Suitable operating environments The Liposat ® power is suitable for environments in the following areas: Professional healthcare facilities with specific requirements Clinics (rooms in A+E, hospital rooms, intensive care, operating theatres, except for in the proximity of active facilities of RF surgery devices or outside of the RF- shielded room for magnetic resonance imaging, first aid facilities). -
Page 21: Starting Up The Liposat Power
® Liposat power Assembly and start-up ® Starting up the Liposat power When installing the Liposat ® power, ensure that: a sufficient distance from other devices is maintained. The Liposat ® power requires a space of at least 30 cm in height and width. ... - Page 22 ® Liposat power ® Liposat power configuration When setting up the device combination with the Vacusat ® power surgical suction device, please proceed in the given order: Take the Liposat power tumescence pump out of its packaging and place it on an ®...
- Page 23 ® Liposat power Assembly and start-up The "Liposat ® power” tumescence pump should be used only with the sterile tube set from Möller Medical, “Liposat power tube set for ® tumescence infiltration - REF 00002251". In order to push the sodium chloride solution through the tube system, the tube must be inserted into the pump.
-
Page 24: Alarms And Service Personnel
® Liposat power ® Liposat power configuration Alarms and service personnel Alarm conditions Alarm 1: Rotor cover opened Physiological alarm condition None Technical alarm condition Opening sensor of the cover activated Alarm limit None Alarm delay < 1 s Alarm signal delay <... -
Page 25: Assistance With Problems
® Liposat power Assistance with problems Assistance with problems ® The Liposat power should not be opened by the user! The user should not use any tool to work on the product! In this chapter possible operating disturbances will be dealt with that might occur with the Liposat ®... - Page 26 ® Liposat power ® Liposat power configuration Set amount does not The set amount is recorded and evaluated through the correspond to the proportional revolution speed of the pump. actually delivered If the set amount does not correspond to the requested amount, amount.
-
Page 27: Service Station
® Liposat power Service station Service station If these steps are not successful, the device must be technically checked. For this purpose, contact the manufacturer or a specialist dealer authorised by it. Before disposal or return of the Liposat ® power, an appropriate disinfection procedure must rule out any possible risk of infection. -
Page 28: Cleaning And Care
® Liposat power ® Liposat power configuration Cleaning and care Cleaning and disinfection No humidity must be allowed to enter into the device. Before cleaning and disinfecting the device surfaces, disconnect the mains plug. Use a lint-free, soft cloth for cleaning and disinfecting. The device is cleaned with a wet cloth in the form of a "scour-wipe-disinfection". -
Page 29: Disposal
® Liposat power Cleaning and care Disposal The present device contains material which must be disposed in accordance with environmental regulations. This device is subject to the European Directive 2012/19/EU on Waste Electric and Electronic Equipment (WEEE2). The identification plate of the device bears the symbol of the crossed through garbage bin. -
Page 30: Accessories And Consumables
® Liposat power ® Liposat power configuration Accessories and consumables Heating dishes for Liposat ® power Order no.: 00002542 (Two items with attachment kit for Vacusat power) ® Dimensions of the heating dishes with packaging: (L x W x H) 540 mm x 300 mm x 300 mm Weight: approx. -
Page 31: Electromagnetic Compatibility
® Liposat power Electromagnetic compatibility Electromagnetic compatibility Electromagnetic emissions The Liposat ® power is suitable for use in the stated electromagnetic environment. Customers and/or operators of the Liposat power should ensure that they use the ® Liposat ® power in such an environment. Measurement of emitted Level of Electromagnetic environment guidelines... - Page 32 ® Liposat power ® Liposat power configuration Immunity test / IEC 60601 - testing Electromagnetic Compliance level standard level environment guidelines The quality of the supply ±1 kV differential mode ±1 kV differential mode voltage should be Surges voltage voltage comparable to that for a IEC 61000-4-5 typical shop or hospital...
-
Page 33: Recommended Safety Distances
® Liposat power Electromagnetic compatibility Immunity test / IEC 60601 - Compliance Electromagnetic environment guidelines standard testing level level Portable and mobile radio transmitting 3 Veff devices, including the cables, should be used 150 kHz to 3 Veff ® in proximity of the Liposat power within the 30 MHz recommended safety distance calculated... -
Page 34: Liposat ® Power Configuration
® Liposat power ® Liposat power configuration Liposat power configuration ® Liposat ® power configuration is the complete medical system for local anesthesia with tumescent solution, consisting of: power – medical infiltration pump for tumescence solution Liposat ® power –... - Page 36 Revision 2020-07 S Software version 019.01.05 Order number for Instructions for use (REF) 20007191 Möller Medical GmbH Wasserkuppenstraße 29-31 36043 Fulda, Germany Tel. +49 (0) 661 / 94 19 5 – 0 Fax +49 (0) 661 / 94 19 5 – 850 www.moeller-medical.com info@moeller-medical.com...








Need help?
Do you have a question about the Liposat power and is the answer not in the manual?
Questions and answers