Table of Contents
Advertisement
Quick Links
TM
Endo PAT
x System
Operation Manual
Itamar Medical REF OM1695230
This product and/or method of use, is covered by one or more of the following US patents: 6319205,
6322515, 6461305, 6488633, 6916289, 6939304, 7374540, as well as any pending US patent
applications and corresponding patents and/or applications filed in other countries.
TM
EndoPAT
, EndoScore™ and PAT™ are trademarks of Itamar Medical, Ltd.
Copyright 2019-2020 by Itamar Medical Ltd.
Advertisement
Table of Contents

Summary of Contents for Itamar Medical EndoPAT Series
- Page 1 6322515, 6461305, 6488633, 6916289, 6939304, 7374540, as well as any pending US patent applications and corresponding patents and/or applications filed in other countries. EndoPAT , EndoScore™ and PAT™ are trademarks of Itamar Medical, Ltd. Copyright 2019-2020 by Itamar Medical Ltd.
- Page 2 LICENSE AGREEMENT IS PROHIBITED. DISCLAIMER Itamar Medical Ltd. shall not be held responsible in any manner for any bodily injury and/or property damage arising from operation or use of this device other than that which adheres to the instructions and safety precautions contained herein and in all supplements...
- Page 3 Itamar Medical Ltd. Record of Editions Edition Date Description Chapter Pages 26 Aug 2019 Initial release 28 Dec 2020 Updated Standards 10-12 Updated installation 20-21 Updated screenshots 23-42 Safety Precautions License agreement link Appendix A Room temperature range 10.5 Label 10.4.1...
-
Page 4: Table Of Contents
Itamar Medical Ltd. Table of Contents General Information ..............1 Intended Use of the EndoPAT x Device ............. 1 Performance and clinical study information ..........1 Equipment Classification ................3 Manufacturers Notice ..................3 Restrictions for Use ..................3 Quality Assurance System: EN ISO 13485 ........... 4 Conventions Used in this Manual .............. - Page 5 Itamar Medical Ltd. Conducting an EndoPAT x Study ........... 47 Pre-Study ......................47 Patient and System Setup ................49 Performing the Study ..................50 Review and Analysis ..............54 Study Data Retrieval ..................54 Automatic Analysis..................54 Study Report ....................55 EndoPAT x study results ................
- Page 6 Itamar Medical Ltd. List of Figures Figure 1 Typical set-up ....................11 Figure 2 – EndoPAT™x System ................... 12 Figure 3 - EndoPAT Study Main Screen ....... Error! Bookmark not defined. Figure 4 - Warning and Error Indications ..... Error! Bookmark not defined.
-
Page 7: General Information
Itamar Medical Ltd. 1 General Information This operation manual is part of the EndoPAT x system. Intended Use of the EndoPAT x Device The EndoPAT x device is a non-invasive device, intended for use as a diagnostic aid in the detection of coronary artery Endothelial Dysfunction (positive or negative) using a reactive hyperemia procedure. - Page 8 Itamar Medical Ltd. The Gold Standard for Endothelial Dysfunction evaluation, the Intra-coronary Acetylcholine (Ach) Challenge method, is routinely performed at the Mayo Clinic. According to the Intra-coronary Acetylcholine (Ach) Challenge method, a catheter is positioned in the origin of the left main coronary artery and Ach is infused with incremental concentration followed by coronary angiogram.
-
Page 9: Equipment Classification
The information in this document is subject to change without notice. Itamar Medical Ltd. makes no warranty of any kind on this material, including but not limited to, the implied warranties of merchantability and fitness for a particular purpose. Itamar Medical Ltd. -
Page 10: Quality Assurance System: En Iso 13485
The device is not intended as a screening test in the general patient population. Itamar Medical Ltd. makes no representation whatsoever, that the act of reading this Operation Manual renders the reader qualified to operate, test or calibrate the system. - Page 11 Itamar Medical Ltd. STANDARD Medical electrical equipment – Part 1-2: General requirements EN 60601-1-2:2015 for basic safety and essential performance - Collateral [the product was standard: Electromagnetic compatibility - Requirements and developed according to: tests IEC 60601-1-2: 2014 (Ed.4)] Medical Device Software – Software Life Cycle Processes...
- Page 12 Itamar Medical Ltd. STANDARD Medical electrical equipment - Part 1-6: General requirements EN 60601-1-6:2010 for basic safety and essential performance - Collateral [the product was standard: Usability developed according to: IEC 60601-1-6:2010 + AMD1:2013] Medical devices - Application of usability engineering to...
-
Page 13: Conventions Used In This Manual
Itamar Medical Ltd. Conventions Used in this Manual The following conventions are used throughout this manual: Warnings Are used to identify conditions or actions, which - if the instructions are ignored - may violate patient safety, or could cause damage/malfunction of the system, resulting in the irretrievable loss of data. - Page 14 Itamar Medical Ltd. Use of an inappropriate cable may cause irreparable damage to the device and may compromise patient safety or electro-magnetic compliance. WARNING The EndoPAT x device should only be installed with and connected to computer equipment that complies with IEC 60950-1 or IEC 62368-1.
- Page 15 The system contains no user-serviceable parts. It should be maintained and serviced only by qualified service personnel, authorized by Itamar Medical Ltd. Purchasers of the EndoPAT x device should ensure that only suitably trained, qualified personnel are authorized to operate the equipment.
-
Page 16: System Overview
Itamar Medical Ltd. 2 System Overview The EndoPAT x device is a computer-based system for non-invasively assessing vascular ™ endothelial dysfunction. It is based on the use of Peripheral Arterial Tone (PAT ) signal technology, during a clinically established procedure, which measures post-ischemic vascular responsiveness following upper arm blood flow occlusion. -
Page 17: Installing The System
Itamar Medical Ltd. 3 Installing the System Basic System Configuration The EndoPAT x device is supplied as a complete package comprising the following components: One EndoPAT x device One EndoPAT x software CD Two pneumo-electric tubing ... -
Page 18: System Description
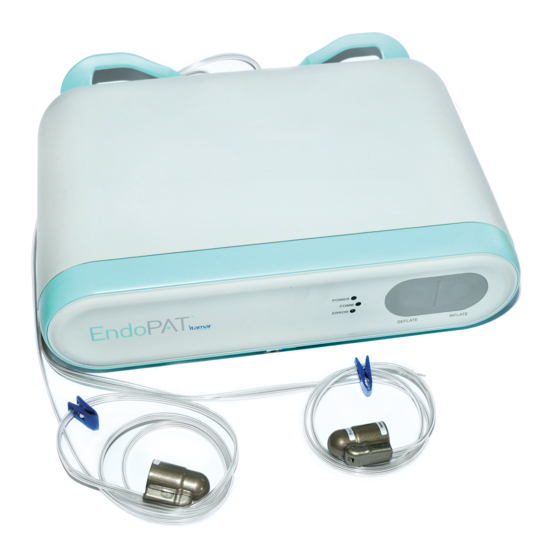
Itamar Medical Ltd. System description The EndoPAT x device front panel has the following indicators (see Figure 2 – EndoPAT™x System): POWER LED indicator COMM LED indicator for the device-computer communication status ERROR LED indicator for error status ... -
Page 19: Connecting The Endopat Tm X Device To The Computer
Itamar Medical Ltd. Connecting the EndoPAT x device to the Computer NOTE The EndoPAT x system requires the use of a USB cable (supplied with the system) for connecting to the computer. 1. Place the EndoPAT x device and computer on a stable platform in close proximity to the examination bed or chair. -
Page 20: Endopat
Itamar Medical Ltd. EndoPAT x Software Installation NOTE Prior to software installation, verify that you are in full system administrator mode with full privileges. Otherwise, the installation might not succeed and could cause operational problems. 1. Close all open applications operating on the computer, including background applications, before installing the EndoPAT x software. -
Page 21: Uninstalling Endopat Tm X Software
Itamar Medical Ltd. 9. When screen appears press “No” (don’t confirm restart!) 10. When the below screen appears click on “Summary” 11. Then you should see “Itamar EPx was successfully installed” 12. Click on the small arrow on the right corner and choose “Quit”... -
Page 22: Shutting Down The System
Itamar Medical Ltd. Shutting Down the System a. Shut down the EndoPAT x software program by clicking on the “X” on the upper right corner. b. Switch OFF the EndoPAT x device using the on/off switch on the back panel. -
Page 23: Endopatx Application Description
Itamar Medical Ltd. 4 EndoPATx Application Description The following screens describe the full application. Viewer mode has the relevant subset of the controls (the title of the screen might be different). EndoPATx User Roles There are three user roles for the EndoPATx:... -
Page 24: Search For Patient Screen - Operator
Itamar Medical Ltd. Figure 5 - Local admin Pull down menu Select Users, a list of existing users will appear and an option to Create Users. Select Create User, the following screen will appear Figure 6 - User screen (Local Admin) - Page 25 Itamar Medical Ltd. Figure 7- Create User Insert user’s information and provide him with a single role (single role per user name). Select Save. Provide the user with their User-name and password. NOTE The system does not send an automatic email to the user, the Local Admin should provide the name and password to each user created.
- Page 26 Itamar Medical Ltd. may select the patient from the drop-down list or click on Search button to open a search window, listing the patients that match the entered details. Figure 9 - Selecting Name/ID From List Figure 10 - Search Button List Selecting a patient either from the drop-down list or Search results opens the Patient Record.
- Page 27 Itamar Medical Ltd. Figure 12 - New Patient When form is filled, click “Save and Continue” button to open Patient Record screen. 4.4.3 Patient Record Screen - Operator The Patient Record Screen displays the personal details and risk factors (optional). In addition Previous Test Records can be accessed through this link.
- Page 28 Itamar Medical Ltd. Figure 13 – Patient Record and New Study 4.4.3.1 Update/Edit Patient details. Clicking on UPDATE on the personal details sections opens the following pop-up window. Click Apply to update the details and return to the Patient Records screen.
- Page 29 Itamar Medical Ltd. Note Patient ID cannot be modified and updated. 4.4.3.2 Update/Edit patient Risk Factors To review and update the risk factors click on the Risk Factors link through Patient Information and New Study window (Figure 13). Select Method: Reynolds, Framingham, European Figure 15 –...
- Page 30 Itamar Medical Ltd. Figure 16 - Risk Factors -European 4.4.3.3 Access previous patient EndoPATx test records. To access previous Patient EndoPATx test records, click on Previous Test Records in Patient information and new Study window (Figure 13). Figure 17 - List of Previous Studies...
- Page 31 Itamar Medical Ltd. Figure 18 - Previous Study Options 4.4.3.4 View Signal Selecting View Signal will open the previous study and enable the auto analysis of the data. Figure 19 - View Signal Screen Pressing the Magic Wand Icon will generate the RHI; LnRHI and HR.
-
Page 32: Conducting Endopatx Study - Operator
Itamar Medical Ltd. Figure 20 -Exported EndoPATx File 4.4.3.6 View Report Selecting View Report will create an automated report in HTML format. See below (Figure Figure 21 - Report File Once the report is opened it can be saved as pdf or sent to print. - Page 33 Itamar Medical Ltd. Figure 22 - Initiating New Test When data insertion is completed, click Start New Test button to start the EndoPAT study. The following window will open. Figure 23 - Open Probs Follow the instructions in section 5 Preparing for a Study and section 6 Conducting an EndoPATTMx Study for detailed patient hookups.
- Page 34 Itamar Medical Ltd. 4.5.2 EndoPATx Study - Main Screen Figure 24 - New Study Screen The main screen of EndoPATx study includes the following main sections: System stability o Indication of leakage detection PAT Signal Waveform Viewer o Display the acquired PAT signals from Probe A and B o Separate gain for each signal .
- Page 35 Itamar Medical Ltd. o Stop the study in middle of test if needed. o Navigation between study stages o Inflate and Deflate buttons for patient setup 4.5.3 Standby Stage Once probes are properly connected (see section Preparation of fingers and hands...
- Page 36 Itamar Medical Ltd. When Baseline Stage timer reaches the recommended time, the Occlusion Stage icon becomes enabled ( ) allowing the transition to Occlusion Stage. Baseline recommended time is 6 minutes. Just before end of Baseline stage and before inflation of the blood pressure cuff, press the Max Gain button on the designated occluded signal.
- Page 37 Itamar Medical Ltd. If needed, increase pressure in the blood pressure cuff to improve occlusion quality. Occlusion Stage timer ( ) is displayed, displaying time elapsed since the stage started and the recommended time for this stage. If stage elapsed time exceeds the recommended time, a “+”...
- Page 38 Itamar Medical Ltd. Figure 26 - End of Study Comment 4.5.7 Post-test study screen and automated analysis. Insert comments if needed and Press Save and Continue. The Patient file will be displayed on the screen. Figure 27 - Study Screen...
- Page 39 Itamar Medical Ltd. 4.5.8 Stopping the Study At any point during the study you may click on the Stop Test button ( This will automatically release the air pressure and deflate the probes. Stopping a test at any point after “Standby” will stamp the probes as “used” and new probes should be attached if restarting the study.
- Page 40 Itamar Medical Ltd. 4.5.10 Warning and Error Indications When an un wanted system condition is detected, the user is provided with error / warning indication at the bottom of the screen. Figure 28 - Error Indicator Clicking on either error tooltip or error indication open the following error report:...
- Page 41 Itamar Medical Ltd. Symbol Severity Sever error condition that impacts study execution Minor/moderate error condition that might impact study execution if not handled in timely manner The time in which the error condition was detected is indicated. Endo PAT x Device...
-
Page 42: Reviewing The Endopatx Study - Reviewer
Itamar Medical Ltd. Reviewing the EndoPATx Study – Doctor (Reviewer) This section is applicable only to a user with the Doctor role. 4.6.1 Locating EndoPATx Study for review Figure 30 - Search Patient- Doctor View Enter the patient name or identifier (ID). The system automatically displays the drop-down list of patients that match the entered the name / ID after typing 3 letters (case sensitive). - Page 43 Itamar Medical Ltd. Figure 32 - Patient Record – Doctor View Clicking on a specific study will open the following pop up screen Figure 33 – Doctor - Single Study Review Options Single study review includes the following options: View Signal: Review signal and edit occlusion borders (if needed).
- Page 44 Itamar Medical Ltd. Figure 34 - Patient Record – Patient View 4.6.3 Study PAT Signal Review Screen The main screen of EndoPATx study review includes the following main sections: PAT Signal Waveform Viewer o Display the acquired PAT signals from Probe A and B...
- Page 45 Itamar Medical Ltd. o Separate gain control for each signal o Study stage bookmarks o Time scale zoom o Timeline marker Editing Toolbar o Undo and Redo last change o Wand tool to automatically define study stages o Eraser to exclude selected section of PAT signal ...
- Page 46 NOTE To enable the Manual Analysis functions, it is necessary to enable the Manual Research Mode by Itamar Medical technician 4.6.5.1 Marking Segments and Artifacts Tool bar icons provide quick and easy access to the tools used to mark segments and artifacts in research studies, as well as to facilitate automatic ratio calculations between PAT™...
- Page 47 Itamar Medical Ltd. 3. Drag the cursor horizontally along the interval—the selected segment becomes highlighted. 4. Release the mouse button at the end of the desired interval—the selected segment remains highlighted and headline indicates its type 4.6.5.2 Analyzing PAT™ Ratios...
- Page 48 Itamar Medical Ltd. 4.6.6 Printing EndoPATx Study Report Select the Print option on the browser to print the study report. Figure 36 - Print Report Through Browser Menu Endo PAT x Device Operation Manual...
-
Page 49: Preparing For A Study
Itamar Medical Ltd. 5 Preparing for a Study Preparing the System for a Study Accessories that are required beside the EndoPAT x system: A set of two PAT probes and anchors Blood pressure cuff (capable of sustaining high pressures for 5 minutes) ... -
Page 50: Creating/Updating Patient's Record
Itamar Medical Ltd. Figure 39 - Press to release Figure 40 - Probe disconnected Creating/Updating Patient’s Record 1 Search for existing Patient (4.4.1) or create a new patient record (4.4.2). 2 Update or enter current patient details as follows: All mandatory fields are marked with asterisk and must be filled in order to proceed to the next step. - Page 51 Itamar Medical Ltd. NOTE All text fields should be entered using text letters and numbers. 3 Enter/update Patient’s Risk Factors - Select the risk factor method (Framingham Risk Score, SCORE Risk or Reynolds Risk Score) and fill in the additional inputs required to calculate Cardio-vascular Risk Factor.
- Page 52 Itamar Medical Ltd. Required in Parameter Remark Framingham Reynolds European Gender* Taken from the patient information main dialog box Systolic blood pressure* Table 1 – Risk Factors mandatory fields * Parameters that are used in the calculation of the Risk, but are part of the main Patient Information Dialog and not the Risk Factors Dialog.
-
Page 53: Conducting An Endopat
Itamar Medical Ltd. 6 Conducting an EndoPAT x Study Pre-Study WARNING Use the blood pressure cuff-set provided by Itamar Medical for arm occlusion. Usage of non-certified cuff- might compromise the results. 6.1.1 General description The EndoPAT system is comprised of a system console and two independent sensing probes coupled to connecting pneumo-electric tubing and foam finger mounting rings. - Page 54 Itamar Medical Ltd. The patient should then be comfortably seated or allowed to lie down in the study room and relax for at least 15 minutes or a sufficient period to reach a relaxed cardiovascular steady- state and to adjust to the room temperature.
-
Page 55: Patient And System Setup
Itamar Medical Ltd. Patient and System Setup 6.2.1 Study conditions The study should be conducted in a quiet and relaxed atmosphere. Phones, beepers and other devices which can cause startling noises should be turned off; otherwise the startle effect on the patient might affect the test result. -
Page 56: Performing The Study
Itamar Medical Ltd. Figure 41 - Applying the PAT™ probes Make sure the tubing forms a loop from the probe, back to the anchor as shown inFigure 41. Gently tape the tube to the tip of the anchored finger, over the finger-nail (Figure 41). - Page 57 Itamar Medical Ltd. NOTE Do not change the time or date of the computer during the study. Changing the windows time while recording might result in corrupted study. 6.3.1 Recording baseline Click the STANDBY icon, from the main screen. The system will display the signals from the two PAT™...
- Page 58 Itamar Medical Ltd. Click the BASELINE icon to begin study recording. 6.3.2 Performing arterial occlusion After a stable period of the required baseline signal recording (5-6min.), prepare for the occlusion: 1. Select Max Gain button of the occluded arm (either probe A or probe B)..
- Page 59 Itamar Medical Ltd. Left – complete occlusion Right – incomplete occlusion In both panels the bottom signal is the occluded arm. Figure 43 - Occlusion quality assessment 6.3.3 Post Occlusion period When exactly five minutes have passed, deflate the pressure cuff as quickly as possible.
-
Page 60: Review And Analysis
Itamar Medical Ltd. 7 Review and Analysis During a PAT™ study, recorded signals are viewed in the display window, based on the appearance of the traces, a qualitative evaluation can be performed. However, subsequent review of the study using the special features described in this chapter facilitates a quantitative analysis of the acquired data. -
Page 61: Study Report
Itamar Medical Ltd. Normal EndoScore™: RHI > 1.67 or LnRHI > 0.51 Abnormal EndoScore™: RHI ≤ 1.67 or LnRHI ≤ 0.51 The HR (Heart Rate) is calculated from the PAT™ signals in the baseline region of interest. 7.2.1 Manual Correction of the Occlusion Borders Click the eraser icon in the Editing toolbar to clear all markings from previous analyses. - Page 62 Itamar Medical Ltd. Normal: RHI > 1.67 Abnormal: RHI ≤ 1.67 This index and threshold were used in the validation study presented at section 1.2 of this manual and they reflect endothelial function. The LnRHI is a natural log transformation of the same index, where: Normal: LnRHI >...
-
Page 63: Cardiovascular Risk
Itamar Medical Ltd. Figure 46 – LnRHI distribution in non-selective population The graph presents the distribution function of the LnRHI in the nonselected population. The data used is composed of nearly 10,000 data points collected from different study cohorts worldwide. - Page 64 Itamar Medical Ltd. 7.5.1 Framingham Risk Score Framingham Risk Score estimates the risk of developing hard CHD adverse events (myocardial infarction or coronary death) over a course of 10 years (Adults Treatment Panel III, JAMA 2001). The risk is given in percentages.
-
Page 65: Additional / Research Features
Itamar Medical Ltd. heart attack before age 60 ( genetic predisposition ) on top of the traditional risk factor analysis. Reynolds Risk Score applies only to healthy population without diabetics above the age of 45. Female/Male with diabetics should not be evaluated using Reynolds Risk Score as they are already considered to be a high-risk group for both heart disease and stroke. - Page 66 Itamar Medical Ltd. Figure 47 - AI calculation Lower AI values (including negative results) reflect better arterial elasticity. AI is usually increasing with age, and is higher in female. The AI section in the report includes the AI result relative to large gender matched non-selective population.
- Page 67 (1.5-2minutes) than the RHI/LnRHI. Researchers that want to use this index for research purposes can ask Itamar Medical to enable this index as part of the registration process. Endo PAT...
-
Page 68: Study Printing
Itamar Medical Ltd. Study printing You can print the displayed data at any time during off-line review and analysisby selecting the Print option through Windows pull down menu. Endo PAT x Device Operation Manual... -
Page 69: Maintenance
Only qualified medical personnel should use this equipment. In the event of equipment malfunction all repairs should be executed by qualified Itamar Medical personnel or authorized service agents. Maintenance instructions should be followed closely to avoid unnecessary equipment failure or potential health hazards to the user or patient. -
Page 70: Troubleshooting
Itamar Medical Ltd. 9 Troubleshooting Description Possible Cause Action Switch on the EndoPAT x device. The EndoPAT x device The EndoPAT x device power does not switch on (the is switched off. POWER LED on the Switch off the EndoPAT x device. - Page 71 Itamar Medical Ltd. Description Possible Cause Action Replace pneumatic cable. Faulty pneumatic cable. Make sure the probes are not touched by other Noisy signal Something is in contact with the fingers, that they are not rested on any surface...
-
Page 72: System Error Messages
Signal is too noisy Noisy signal prevents proper operation of the analysis module. Internal Server Error Internal system failure (call Itamar Medical customer support). Baseline duration is shorter Less than 2 min and 20 sec valid baseline signals. than minimum required... -
Page 73: Technical Information
Itamar Medical Ltd. 10 Technical Information 10.1 System Minimum Requirements ® An IBM or compatible PC with dual core processor 1.4 GHZ CPU or higher Internet browsers Chrome version 70, Edge version 44, Firefox version 63 or above versions 8.00 GB RAM, Windows 10 64 bits... - Page 74 Itamar Medical Ltd. (Medical Device Directive) and related standards. The product is marked with the CE logo. Use-by date Single use, do not re-use Temperature limit Medical device Manufacturer Catalogue Number Serial Number According to the WEEE Directive 2012/19/EU, all waste electrical and electronic equipment (EEE) should be collected separately and not disposed of with regular household waste.
-
Page 75: Labeling
Itamar Medical Ltd. 10.4 Labeling 10.4.1 System Label The System Label is located at the bottom of the EndoPAT x device: 10.4.2 Power Rating Label The Power Rating Label is located near the power inlet connector of the EndoPAT device: 10.4.3 Applied Part Label... - Page 76 Itamar Medical Ltd. 10.4.4 Packaging labels The following labels are attached to the master package of the EndoPAT x system: Endo PAT x Device Operation Manual...
-
Page 77: Specifications Of Endopat Tm X System
Itamar Medical Ltd. 10.5 Specifications of EndoPAT x system Properties Description PAT™ Probe Itamar Medical’s proprietary probe only Recording Time Limited by hard disk space, ~35MB per study of 20 minutes Indications 3 LED’s - power, communication, error PAT™ Channel Selective Gain 1÷... -
Page 78: Appendix A: License Agreement And Limited Warranty
The document can be viewed at https://www.itamar-medical.com/wp-content/uploads/2020/09/License-Agreement- 09.2020.pdf Should you have any questions concerning this License Agreement, or if you desire to contact Itamar Medical for any reason, please write to: USA: Itamar Medical Inc. 3290 Cumberland Club Drive, Suite 100... -
Page 79: Appendix B: Regulatory Authorized Representative
Itamar Medical Ltd. Appendix B: Regulatory Authorized Representative Arazy Group GmbH The Squaire 12, Am Flughafen, 60549 Frankfurt am Main, Germany Endo PAT x Device Operation Manual... -
Page 80: Appendix C: Manufacturing Declarations According To Iec 60601-1-2
- before clinical use – to check the equipment for correct operation under the conditions of use. The use of accessories other than those specified or sold by Itamar Medical as replacement parts may have the consequence of increasing the emissions or decreasing the immunity of the unit. - Page 81 Itamar Medical Ltd. Table 2 - from IEC 60601-1-2:2014 Guidance and manufacturer's declaration – electromagnetic immunity – EndoPAT x (EPX) The EDx is intended for use in the electromagnetic environment specified below; The customer or the user of the EPX should assure that it is used in such an environment.
- Page 82 Itamar Medical Ltd. Table 3 - from IEC 60601-1-2:2014 Guidance and manufacturer's declaration – electromagnetic immunity – EndoPAT x (EPX) The EPX is intended for use in the electromagnetic environment specified below; The customer or the user of the EPX should assure that it is used in such an environment.
- Page 83 Itamar Medical Ltd. Table 4 - from IEC 60601-1-2:2014 Recommended separation distances between portable and mobile RF communications equipment and the EndoPATx The EPX is intended for use in the electromagnetic environment specified below; The customer or the user of the EPX should assure that it is used in such an environment.
- Page 84 Itamar Medical Ltd. Test Band a) Service a) Modulation Maximum Distance Immunity Compliance frequency (MHz) power Test level level (MHz) (V/m) (V/m) CDMA 850, LTE Band 5 1720 1 700 – GSM 1800; Pulse modulationb) 1 990 CDMA 1900; 217 Hz 1845 GSM 1900;...
- Page 85 Itamar Medical Ltd. Table 5 - Test specifications for ENCLOSURE PORT IMMUNITY to RF wireless communications equipment Test Band Service Modulati Maximum Distance IMMUNIT Compliance level frequency power (V/m) (MHz) (MHz TEST LEVEL (V/m) 380 – TETRA 400 Pulse modulatio 18 Hz 430 –...
- Page 86 Itamar Medical Ltd. Recommended Separation Distances The EndoPAT x is intended for use in an electromagnetic environment in which radiated radiofrequency disturbances are controlled. The user and/or installer of the unit can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile radiofrequency communications equipment...
-
Page 87: Appendix D: Spare Parts List
Itamar Medical Ltd. APPENDIX D: SPARE PARTS LIST The following items can be ordered and purchased individually: Pneumatic PAT Probe (a box of 12 probes) Pneumo-electric tubing Set of 30 foam finger anchors Accessories Kit ...

Need help?
Do you have a question about the EndoPAT Series and is the answer not in the manual?
Questions and answers