Table of Contents
Advertisement
Quick Links
Download this manual
See also:
Patient Manual
Biomet
®
SpinalPak
®
Non-Invasive Spine Fusion Stimulator System
Complete Manual and Package Insert
100 Interpace Parkway • Parsippany, NJ 07054
800-526-2579 • www.biomet.com • BNS231002L
Non-Invasive Stimulation
©2009 EBI, LLC. All trademarks are the property of Biomet, Inc.
™
OPTIONS • EVIDENCE • EXPERIENCE
or its subsidiaries unless otherwise indicated. Rx Only.
1067795-00 REV C
Advertisement
Table of Contents

Summary of Contents for BIOMET SpinalPak
- Page 1 Complete Manual and Package Insert 100 Interpace Parkway • Parsippany, NJ 07054 800-526-2579 • www.biomet.com • BNS231002L Non-Invasive Stimulation ©2009 EBI, LLC. All trademarks are the property of Biomet, Inc. ™ OPTIONS • EVIDENCE • EXPERIENCE or its subsidiaries unless otherwise indicated. Rx Only.
-
Page 2: Table Of Contents
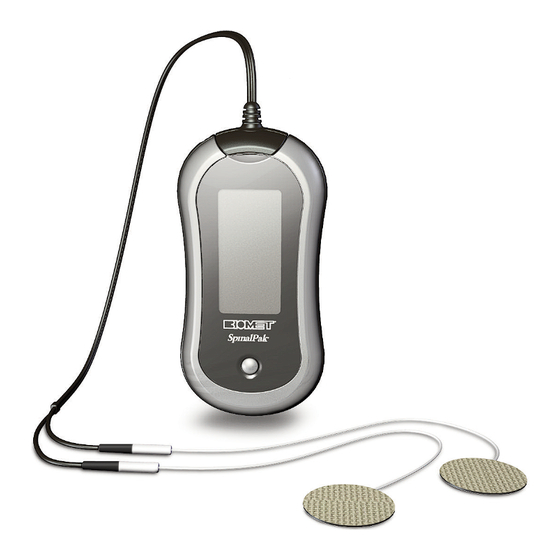
® - LT-4500 (PN 106130-12) • Charger (PN 1067721) IMPORTANT SAFEGUARDS ......................2 • Batteries (2) (PN 1067720) BIOMET ® SPINALPAK ® STIMULATOR ..................3 • Electrode Covers (PN 106130-17) • Stimulator (PN 1067717) • Description ..........................3 • Device Holster (PN 1067722) •... -
Page 3: Important Safeguards
Charger 1-800-822-8837. Input: 88-240 VAC 50/60 Hz 6 W NOTE: Inside the United States call Biomet at 1-800-526-2579 or 1-973-299-9300 if calling from Output: 12V DC 500mA outside the United States with any questions or problems. For use with the Biomet ®... -
Page 4: Device Holster (Pn 1067722)
Note: The first battery pack may not provide a 24-hour treatment initially. It is recommended that the patient keep one WARNINGS ® battery pack in the battery charger to assure that it is fully charged and ready, and the other inserted into the Biomet Cardiac pacemakers or cardioverters may be adversely affected by the Biomet ®... -
Page 5: Directions For Use
See instructions for use on the electrode packet. The patient should consult their prescribing physician or Biomet if they have any questions or concerns regarding proper electrode placement. If their skin becomes abnormally red at the electrode sites, the electrodes should be moved adjacent to the original sites. -
Page 6: Button Functions
Remove the battery pack from the battery briefly to silence the alarm. Depress the charger (Figure 9) and place the fully charged battery button approximately 3 seconds to ® pack into the Biomet SpinalPak ® Stimulator in order engage or disengage the audible alarm. -
Page 7: Treatment Completion
This data may be downloaded to a personal computer for viewing, storage and/or print out via the use of Biomet Compliance Data Download Software. Please call your local Biomet representative to obtain more information. -
Page 8: Electromagnetic Compatibility
Electromagnetic Compatibility Table 2 A. The use of accessories, cables or replacement parts other than those supplied by Biomet Guidance and manufacturers declaration - electromagnetic immunity may result in increased emissions or decreased immunity of the equipment or system. B. This equipment should not be used adjacent to or stacked upon other equipment. - Page 9 The Biomet ® SpinalPak ® Stimulator is intended for use in the electromagnetic radiated RF disturbances are controlled. The customer or the user of the Biomet ® SpinalPak ® environment specified below. The customer or the user of the Biomet ®...
-
Page 10: Patient Counseling Information
Damage may occur if the device is inappropriately handled or abused. Disposal Instructions When treatment has concluded as determined by the prescribing physician (see page 10), Biomet requests that the patient disposes of the Stimulator according to local statutes Biomet ®...




Need help?
Do you have a question about the SpinalPak and is the answer not in the manual?
Questions and answers