Table of Contents
Advertisement
Quick Links
Negative Pressure Wound
Therapy System
extriCARE
Operating Manual
Suite M-Q, 12th Floor, Kings Wing Plaza 2, 1 On Kwan St. Shek Mun, Shatin, N.T., Hong Kong
Tel: +852 3166 7200
United States Contact:
Toll-free line at 1-877-312-NPWT
4709 Distribution Ct #2, Orlando, FL 32822
info@allevamedical.com
3600
®
ENGLISH
MEDICAL CO.
Fax: +852 2355 7663
IFU38.0012 Rev C 20150317
EN-US
www.allevamedical.com
© 2018
Advertisement
Table of Contents

Summary of Contents for Alleva extriCARE 3600
- Page 1 Negative Pressure Wound Therapy System extriCARE 3600 ® Operating Manual ENGLISH IFU38.0012 Rev C 20150317 EN-US MEDICAL CO. Suite M-Q, 12th Floor, Kings Wing Plaza 2, 1 On Kwan St. Shek Mun, Shatin, N.T., Hong Kong Tel: +852 3166 7200 Fax: +852 2355 7663 www.allevamedical.com United States Contact:...
- Page 2 (at least 22 of 24 hours per day). The extriCARE 3600 device is not to be used for home use. Caution: Federal law restricts this device to sale by or on the order of a licensed healthcare...
-
Page 3: Symbol List
2. Symbol List Battery: Full <33%: <10%: Power Adapter: When the device is plugged in and turned on, the screen will display the power adapter icon. During this time, the device will be powered by the external power, and the battery will also recharge. Bell: When an alarm is occurring, the symbol on the right will appear, showing that the alarm is not muted. - Page 4 2. Symbol List (continued) Warning/Caution: See instructions for use for additional guidance Single Use Only Date Of Manufacture Type BF applied part Keep Dry Serial Number Power Switch Manufacture Lot Number Authorized Representative in the European Community Class II Equipment Use By...
- Page 5 Conforms to AAMI STD ES.60601-1, HA 60601-1-11 Certified to CSA STD C22.2 No.60601-1, 60601-1-11 4005914 Manufacturer Catalog / Model Number Sterilized Using Ethylene Oxide Refer to instruction manual. Protected against foreign objects equal to or greater than 12.5mm and against falling drops of water when enclosure tilted up to 15°.
-
Page 6: Device Specifications
3. Device Specifications DIMENSIONS: Length: 6.7” (17cm) Depth: 4.3” (11cm) Height: 5.1” (13cm) WEIGHT: 2.87 lbs (1.3 kg) BATTERY TYPE: Lithium Battery, 10.8V, 4400mAh (rechargeable) AC/DC ADAPTER: Model Number: GTM91099-6015-T2 AC Input: 100-240V, 1.5A, 50/60Hz DC output: 15V 4A, 60W VACUUM MODES: Continuous or Intermittent OPERATING CONDITIONS:... -
Page 7: Contraindications For Use
4. Accessories 1. AC/DC Adapter: Please only use the AC/DC adapter provided in the package. 2. Tubing Set: 1.55m tubing with a luer-lock connector on one end preattached. A clamp is also attached to the tubing. 3. Canister: Available in 400cc and 1000cc. 4. - Page 8 3600 Negative Pressure Wound ® Therapy Pump System. If clarification is needed, contact technical personnel or Alleva Medical Products at 1-866-446-0092 prior to use. Additional questions can be immediately addressed as well. • Do not use the extriCARE 3600 Negative Pressure Wound Therapy Pump around ®...
- Page 9 8. Precautions 8.1) Be aware for any of the following conditions: There are additional conditions to take into account before using Negative Pressure Wound Therapy, such as: BLEEDING: There is a risk of bleeding/hemorrhaging with negative pressure wound therapy. If hemostasis cannot be achieved, if the patient is on anticoagulants or platelet aggregation factors, or if the patient has friable blood vessels or infected vascular anastomosis, he or she may have an increased risk of bleeding;...
- Page 10 8.1) Be aware for any of the following conditions (continued): SPINAL CORD INJURY: If a patient experiences autonomic dysreflexia (sudden changes in blood pressure or heart rate because of sympathetic nervous system stimulation) discontinue extriCARE therapy to minimize ® sensory stimulation and give immediate medical assistance. MODE: In unstable anatomical structures, continuous rather than intermittent therapy is recommended to help minimize movement and instability.
- Page 11 8.2) Prior to Therapy • Patient should be assessed and measures should be taken to optimize and stabilize their medical condition. Nutrition, medication, blood glucose, blood pressure, and circulation as well as other medical issues should be addressed. • The wound should be recently debrided by whatever measure is appropriate and the amount of necrotic tissue should be minimized.
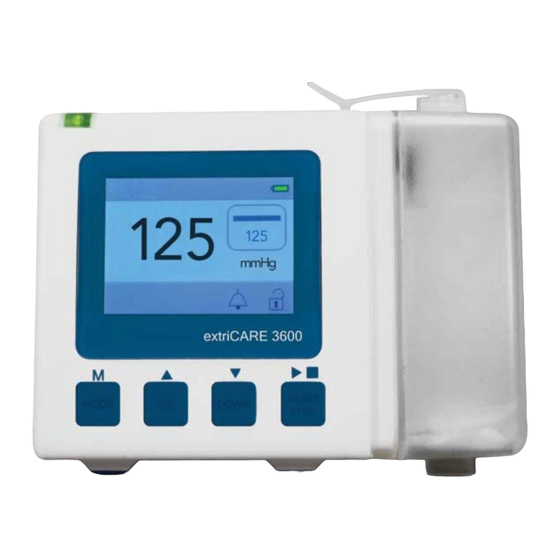
- Page 12 9. Features 9.1) Defined Features 18. Charging Symbol 16. Audio Symbol 17. Pump Symbol 15. Canister Clip 14. Tubing Input 1. Indicator Light 2. Battery Power 12. Mode Symbol 3. Actual Pressure 11. Set Pressure 13. Lock Symbol 10. Canister Error Symbol 4.
- Page 13 9. Features 9.1) Defined Features (continued) 6. Up Button: This increases the set pressure in increments of 5 mmHg up to a maximum of 200 mmHg. 7. Down Button: This decreases the set pressure in increments of 5 mmHg down to a minimum of 40 mmHg.
- Page 14 9.2) Alarm Features/Troubleshooting Error Type Cause/Description Audio Alarm Visual Alarm System Status Suggested Features Features Mitigation Pump Properly The canister is not 3 beeps Canister remains on install the detected or is every 20 Installation canister installed incorrectly seconds Error Yellow LED flashing every 2 seconds...
- Page 15 9.2) Alarm Features/Troubleshooting (continued) Error Type Cause/Description Audio Alarm Visual Alarm System Suggested Features Features Status Mitigation Air Leakage extriCARE 3600 There are many potential sources of leaks (incomplete seal between ® Error dressing and skin, improper connection between tubing, canister leakage, etc.). The alarms have been divided into two categories, mild and severe.
-
Page 16: Instructions For Use
10. Instructions for Use 10.1) Dressing and Canister Application include bandages and foam kits. Follow detailed extriCARE Wound Dressings ® instructions that come with your extriCARE Wound Dressing to apply the ® dressing. The clinician may loosely place extra non occlusive dressing material into areas of undermining and tunneling. - Page 17 10.1) Dressing and Canister Application (continued) When using on a venous or other leg ulcer: • Edema control must continue during wound treatment. • Consider lower pressures when applied over fragile skin. When applying the wound dressings over toes: extriCARE ®...
- Page 18 10.2) Operating the Device (continued) 3. MODE: The extriCARE 3600 can operate in continuous or intermittent modes. ® In continuous mode, the extriCARE 3600 will continuously operate at the ® set pressure. In intermittent mode, the extriCARE 3600 will operate for 5 ®...
- Page 19 10.3) Rail Clamp Rail clamp (Figure 1) is provided to hang the device in case it is necessary. To use the rail clamp, insert the clamp board firmly into the socket located at the back of the device. Release the screw on the clamp to make space for the hanging media. Snap the clamp onto the hanging medium with the socket opening facing the ground.
-
Page 20: Maintenance And Replacement Parts
fire or water. Use only a Alleva Medical Products approved battery. If the device will not be in use for an extended period of time, the battery should be maintained by recharging regularly. -
Page 21: Warranty Information
Operation Manual. THERE ARE NO OTHER WARRANTIES THAN THOSE EXPRESSLY STATED HEREIN. TO THE EXTENT PERMITTED BY LAW, ALLEVA MEDICAL PRODUCTS DOES NOT MAKE ANY IMPLIED WARRANTY OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE AS TO ANY PRODUCT OR DEVICE, WHETHER OR NOT THAT PRODUCT OR DEVICE IS COVERED BY ANY EXPRESS WARRANTY CONTAINED HEREIN. -
Page 22: Product Classification
Appendix 1 Product Classification: • According to the type of protection against electrical shock, this device is classified as a Class II Equipment, and Type BF Equipment that is powered by anexternal electrical power source. • According to the degree of protection against harmful ingress of water this system is classified as Ordinary Equipment (IP22: Protected against foreign objects equal to or greater than 12.5mm and against falling drops of water when enclosure tilted up to 15°.) -
Page 23: Accompanying Documents
Appendix 2 Accompanying Documents: A. Instructions for use needs special precautions regarding EMC and needs to be installed and MODEL 3600 put into service according to the EMC information provided in the ACCOMPANYING DOCUMENTS; 2. Portable and mobile RF communications equipment can affect MODEL 3600 B. -
Page 24: Guidance And Manufacturer's Declaration - Electromagnetic Immunity
Guidance and Manufacturer’s Declaration - Electromagnetic Immunity is intended for use in the electromagnetic environment MODEL 3600 specified below. The customer or the user of the should MODEL 3600 assure that it is used in such an environment. Immunity Test IEC 60601 Test Level Compliance Level Electromagnetic Environment... - Page 25 Guidance and Manufacturer’s Declaration - Electromagnetic Immunity is intended for use in the electromagnetic environment MODEL 3600 specified below. The customer or the user of the should MODEL 3600 assure that it is used in such an environment. Immunity Test IEC 60601 Test Level Compliance Level Electromagnetic Environment...
- Page 26 Recommended separation distances between portable and mobile RF communications equipment and the MODEL 3600. MODEL 3600 is intended for use in an electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of MODEL 3600 can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the MODEL 3600...
- Page 27 References References available upon request.
- Page 28 MEDICAL CO. Suite M-Q, 12th Floor, Kings Wing Plaza 2, 1 On Kwan St. Shek Mun, Shatin, N.T., Hong Kong Tel: +852 3166 7200 Fax: +852 2355 7663 www.allevamedical.com United States Contact: Toll-free line at 1-877-312-NPWT 4709 Distribution Ct #2, Orlando, FL 32822 info@allevamedical.com ©...



Need help?
Do you have a question about the extriCARE 3600 and is the answer not in the manual?
Questions and answers